Method for separating benzaldehyde and nitrobenzaldehyde by employing high performance liquid chromatography
A technology of high performance liquid chromatography and nitrobenzaldehyde, which is applied in the direction of material separation, analysis of materials, and measuring devices, to achieve good accuracy, good peak shape, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: HPLC detection of lercanidipine hydrochloride bulk drug.
[0035] Instrument: Waters e2695-2489 high performance liquid chromatography
[0036] Chromatographic column: Phenomenes ACE Excel 5 C 18 -PFP (250*4.6mm, 5μm)
[0037] Mobile phase: 0.05mol / L dipotassium hydrogen phosphate (add 3ml of triethylamine, adjust the pH to 7.5 with phosphoric acid)-methanol (80:20)
[0038] Diluent: mobile phase
[0039] Detection wavelength: 240nm
[0040] Stationary phase temperature: 40°C
[0041] Flow rate: 1.0ml / min
[0042] Injection volume: 20μl
[0043] Workstation: Empower
[0044] Get 1.0g of lercanidipine hydrochloride bulk drug and add appropriate amount of mobile phase to dissolve and dilute to make lercanidipine hydrochloride 40mg / ml as need testing solution, get benzaldehyde, 2-nitrobenzaldehyde, 3-nitrobenzaldehyde in addition and 4-nitrobenzaldehyde reference substance in appropriate amounts, accurately weighed, added mobile phase to dissolve and...
Embodiment 2
[0047] Embodiment 2: Use this method to examine benzaldehyde and nitrobenzaldehyde in lercanidipine hydrochloride at different flow rates.
[0048] Instruments and reagents are the same as in Example 1, and other chromatographic conditions are the same as in Example 1 except for the flow rate. The flow rates for this study were 0.9 ml / min, 1.0 ml / min and 1.1 ml / min.
[0049] Take 2.0g of lercanidipine hydrochloride crude drug, add benzaldehyde, 2-nitrobenzaldehyde, 3-nitrobenzaldehyde and 4-nitrobenzaldehyde reference substance mother liquor, add appropriate amount of mobile phase to dissolve and dilute to make hydrochloric acid 40 mg / ml of lercanidipine, 3 μg / ml of benzaldehyde and 3 μg / ml of ortho-p-nitrobenzaldehyde plus impurities for the test solution. Take another appropriate amount of benzaldehyde, 2-nitrobenzaldehyde, 3-nitrobenzaldehyde and 4-nitrobenzaldehyde reference substances, weigh them accurately, add mobile phase to dissolve and quantitatively dilute to form ...
Embodiment 3
[0052] Embodiment 3: Use this method to check benzaldehyde and nitrobenzaldehyde in lercanidipine hydrochloride at different column temperatures.
[0053] Instrument and reagent are the same as Example 1, and other chromatographic conditions are the same as Example 1 except column temperature. In this study, the column temperatures were 38°C, 40°C and 42°C.
[0054] Solution preparation is the same as embodiment 2.
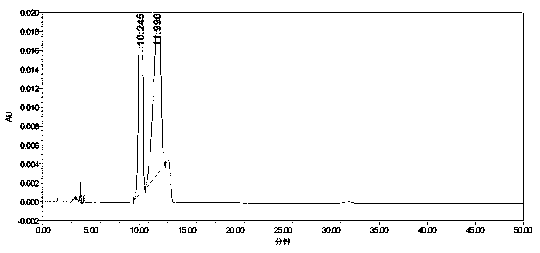
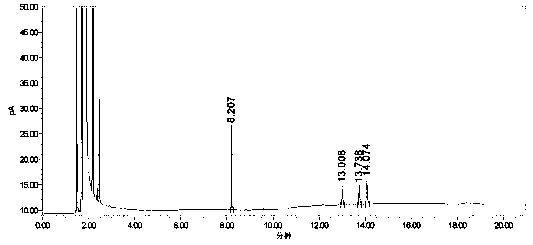
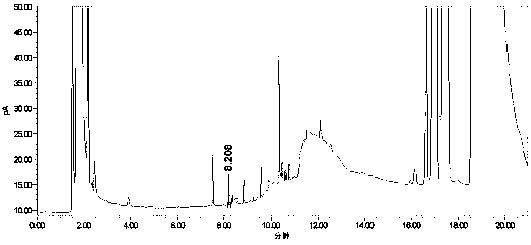
[0055] Take the blank solution (mobile phase), the mixed impurity reference substance solution, and the impurity-added lercanidipine hydrochloride test solution, measure according to the above chromatographic conditions, and record the chromatogram.
[0056] Test results: Calculated by the external standard method, within the column temperature range of 38°C to 42°C, the RSD% value of benzaldehyde content is 4, the RSD% value of o-nitrobenzaldehyde content is 1.4, and the content of m-nitrobenzaldehyde The RSD% value was 0.7, and the RSD% value of p-nitrobenzald...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com