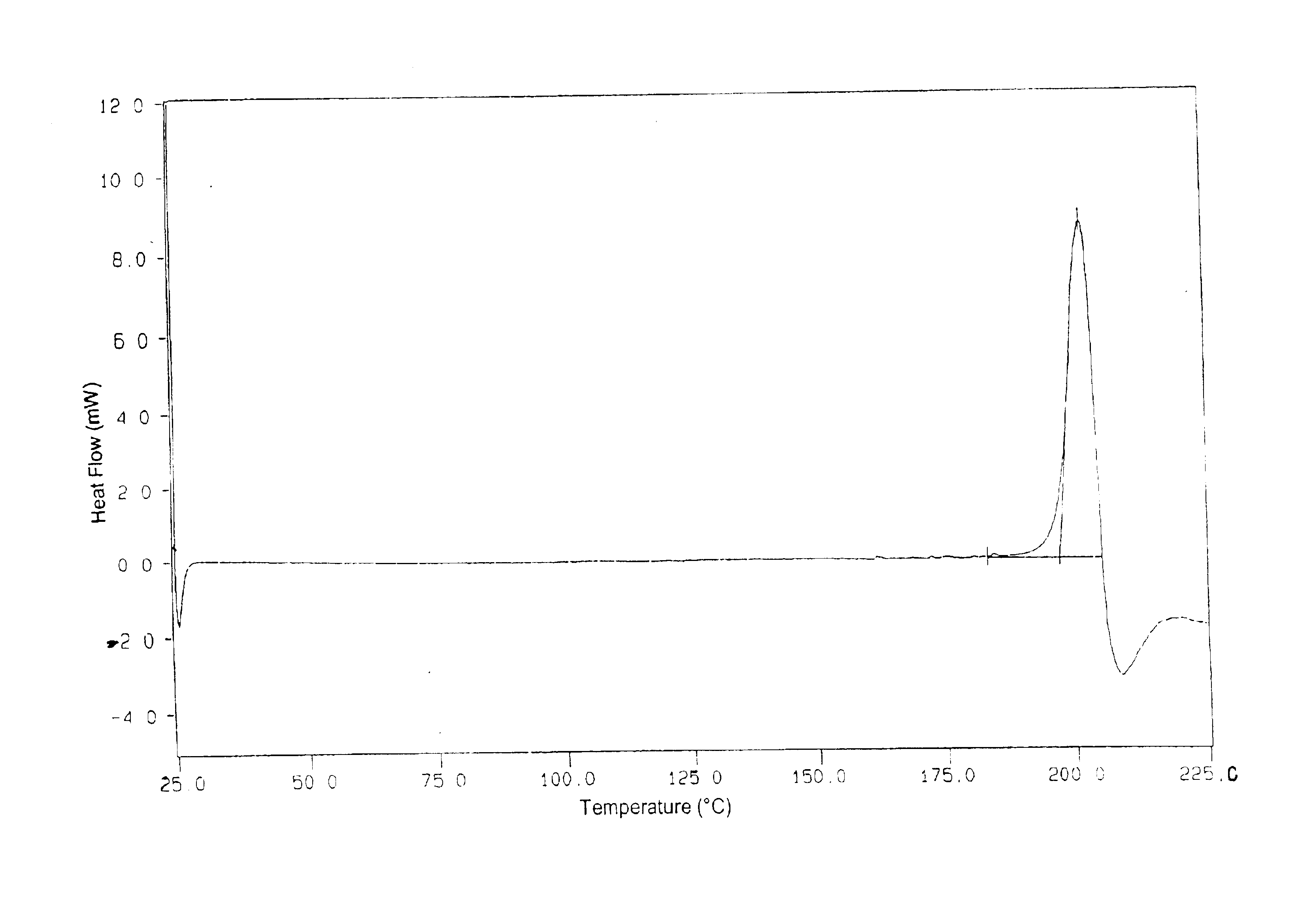
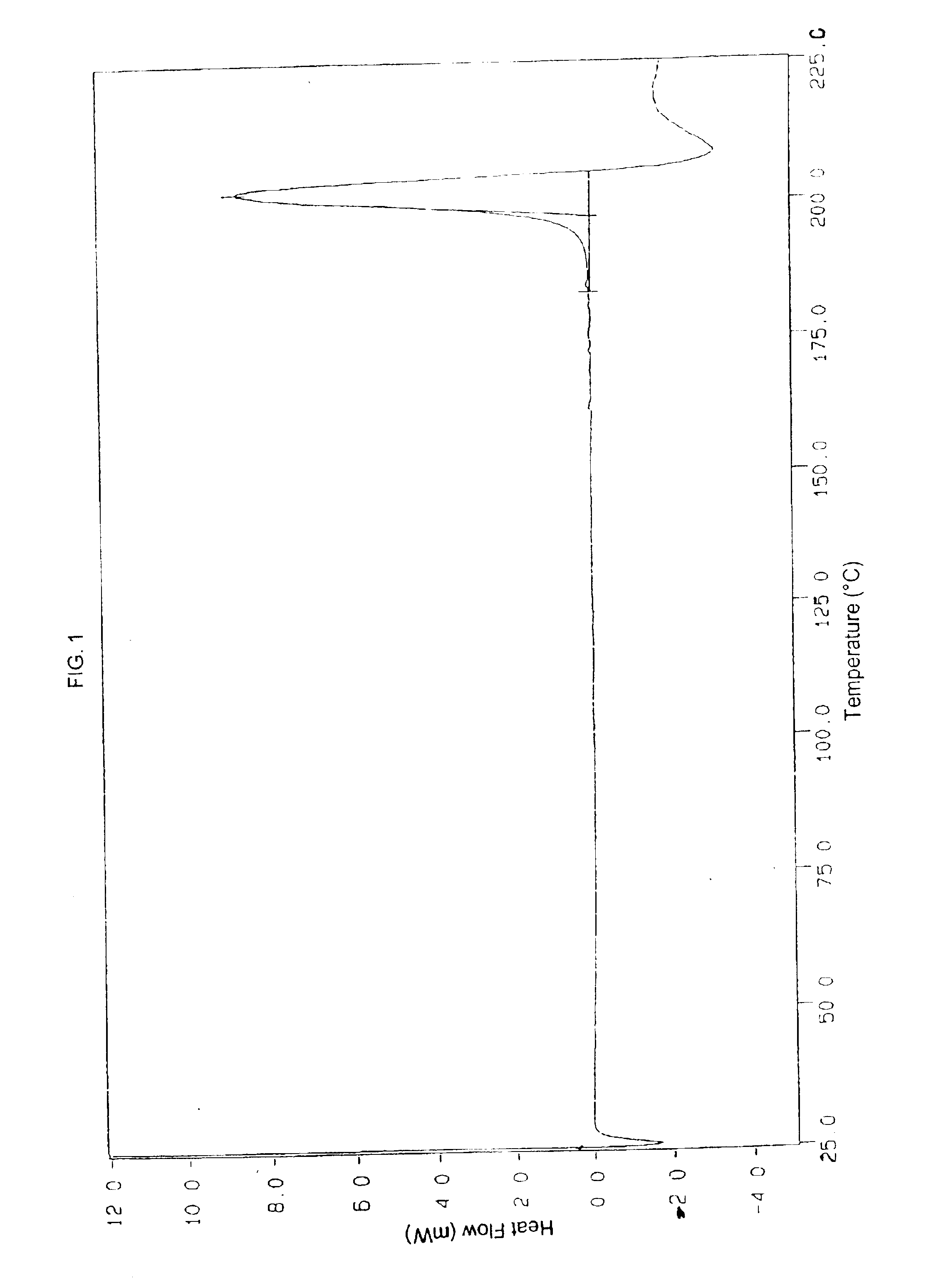
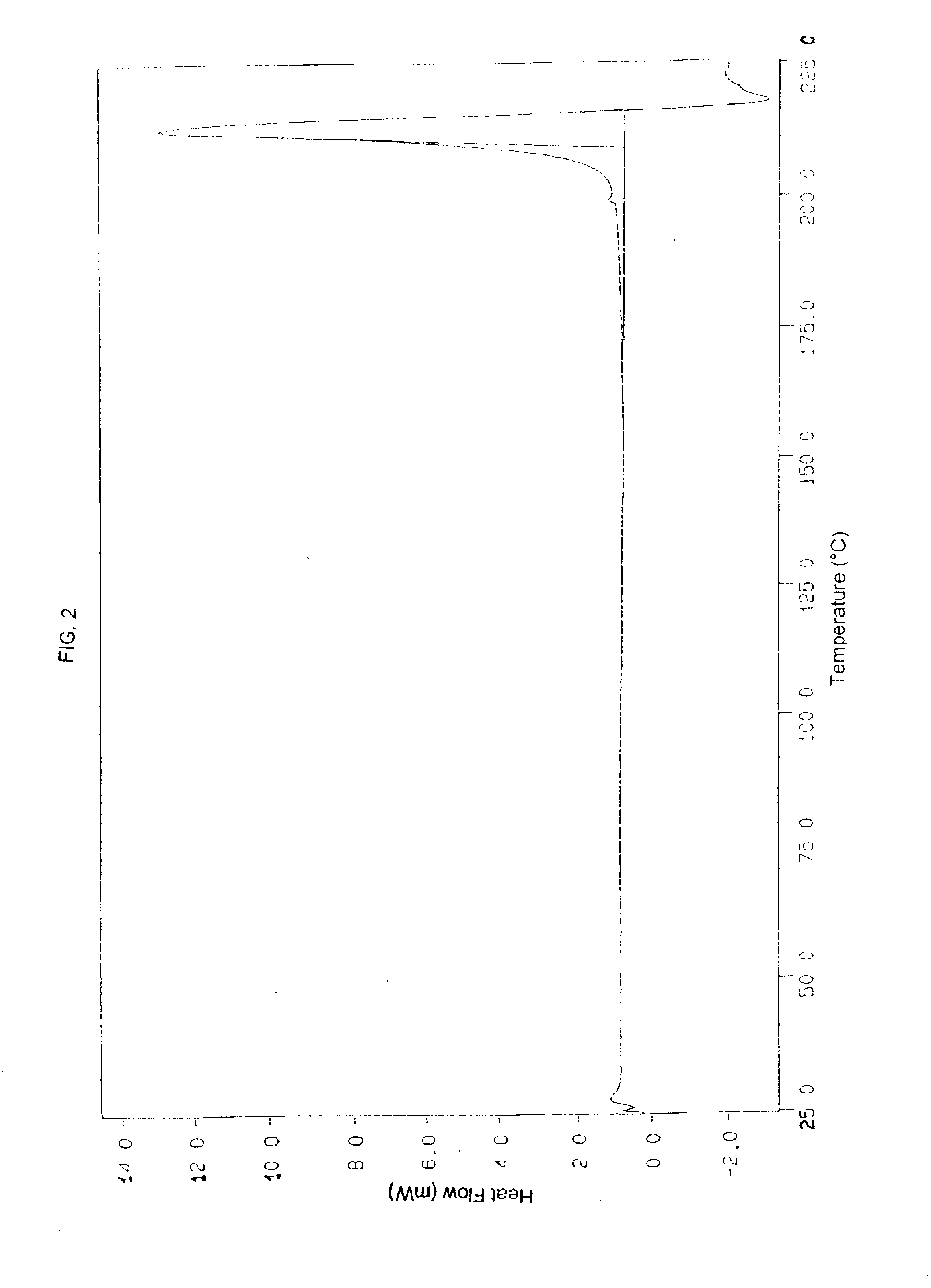
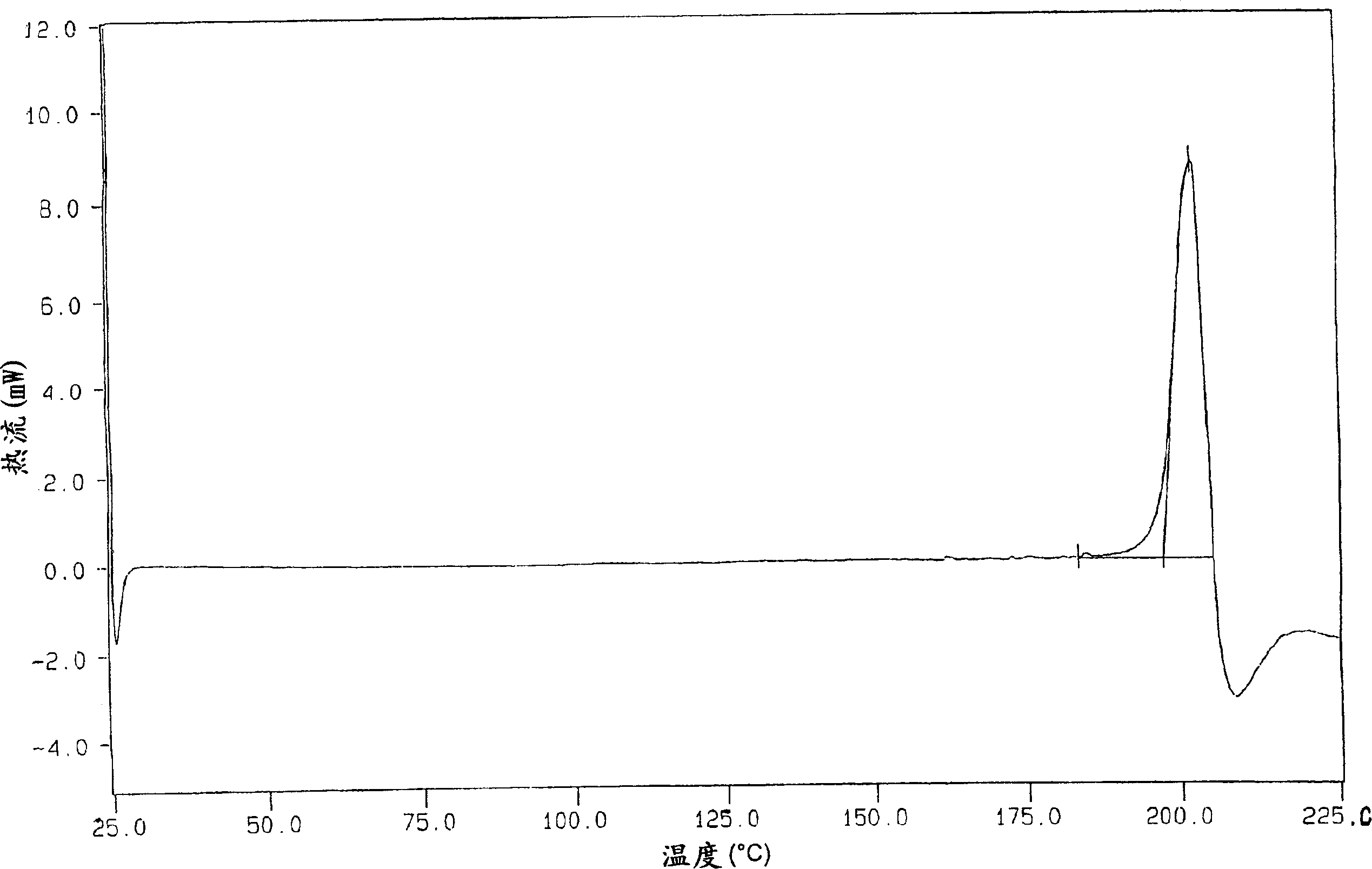
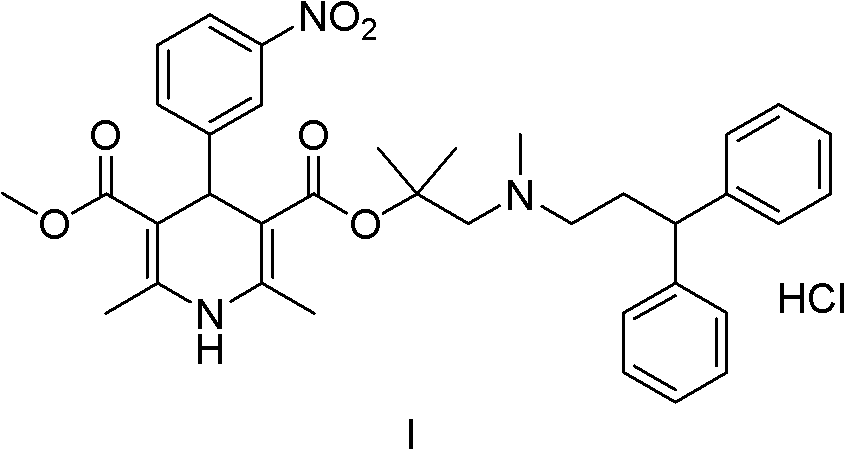
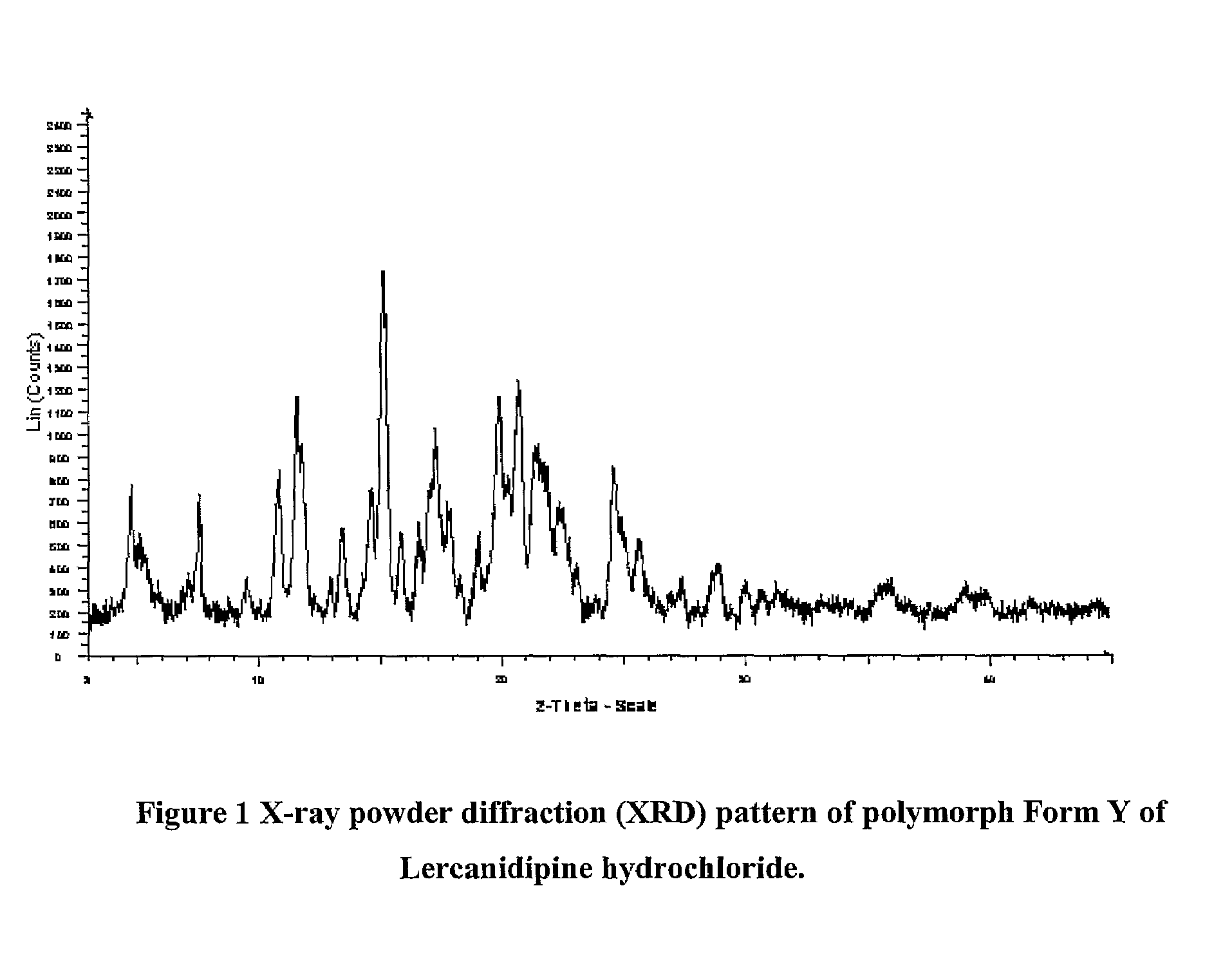
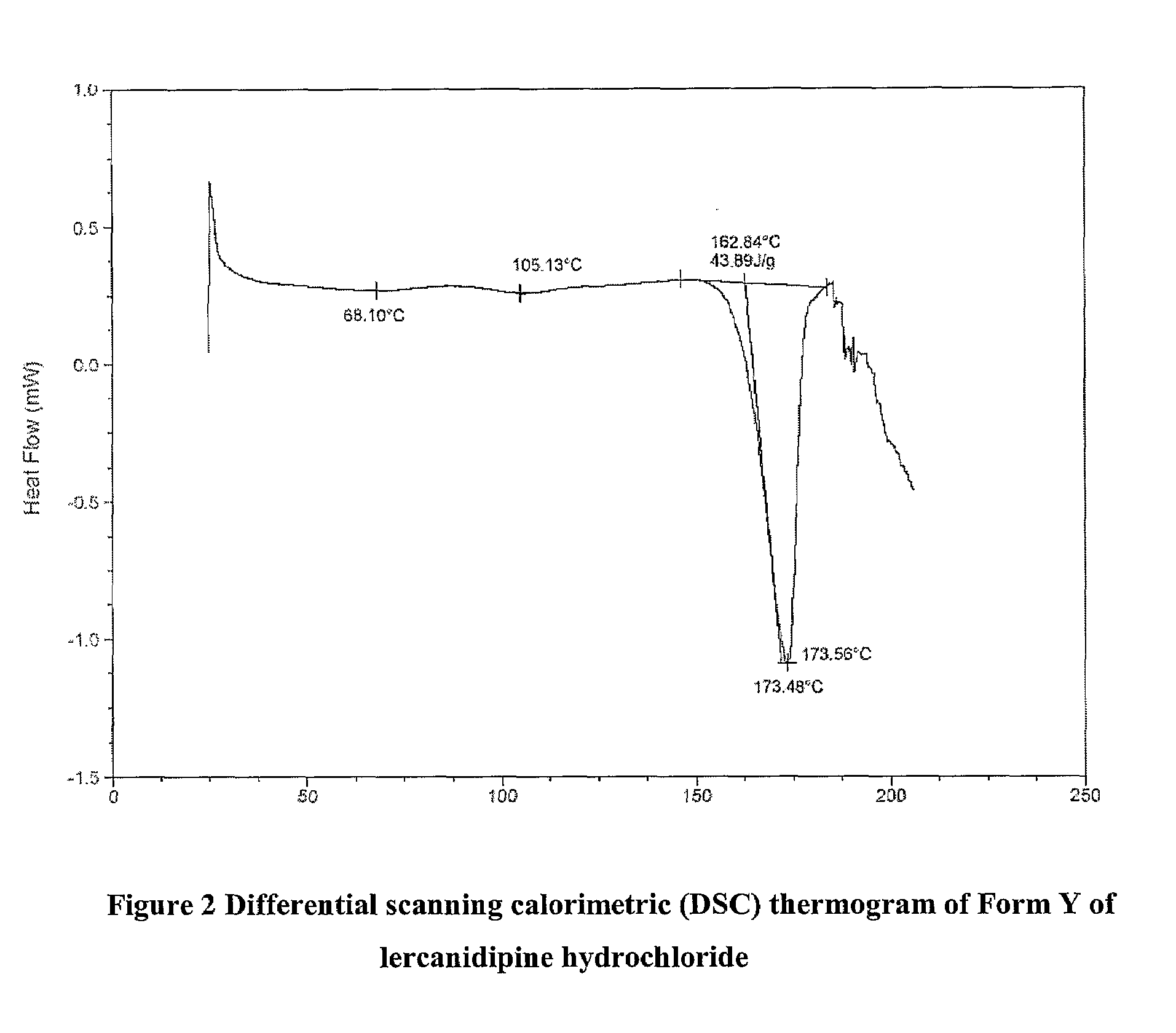
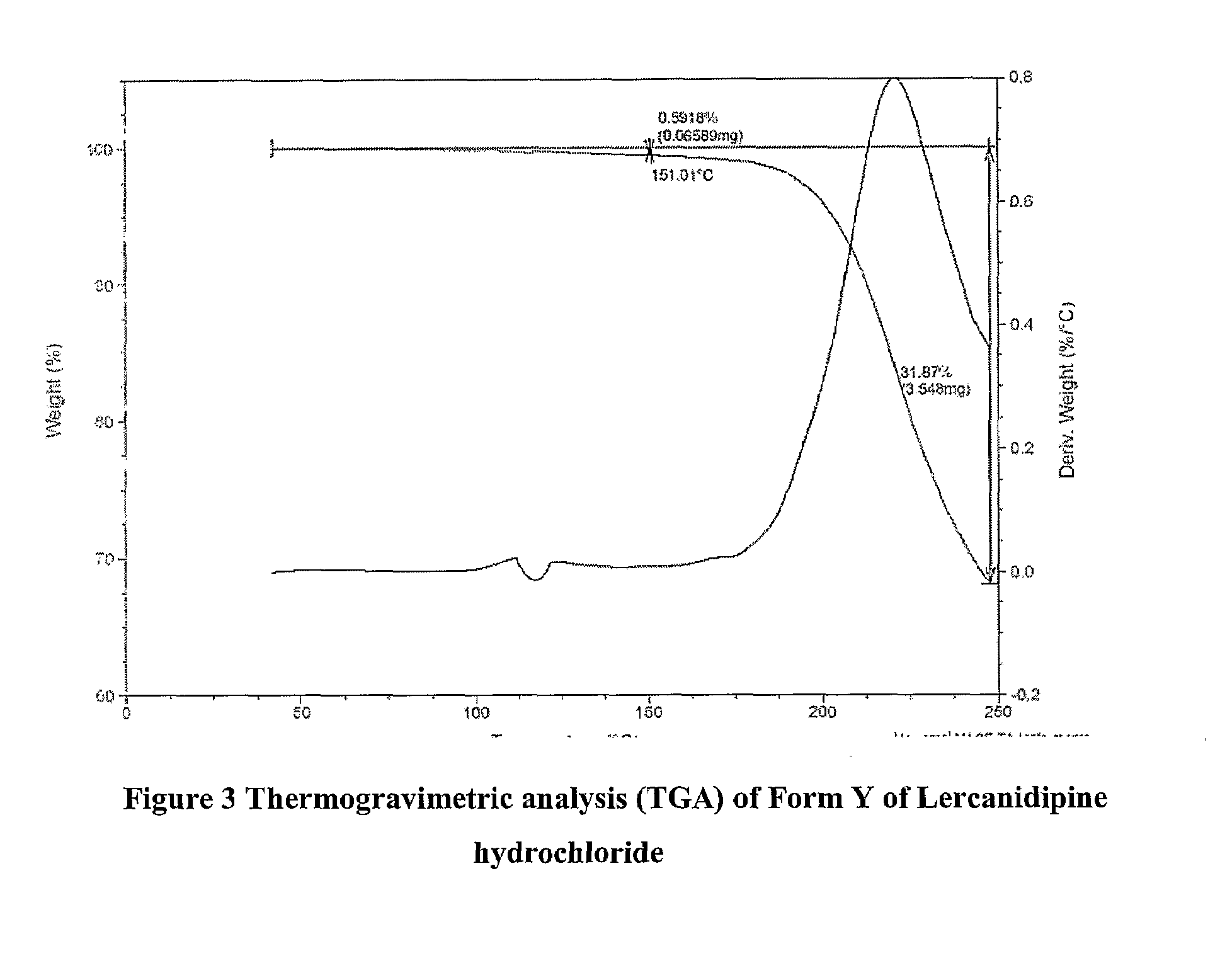
Patents
Literature
37 results about "Lercanidipine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
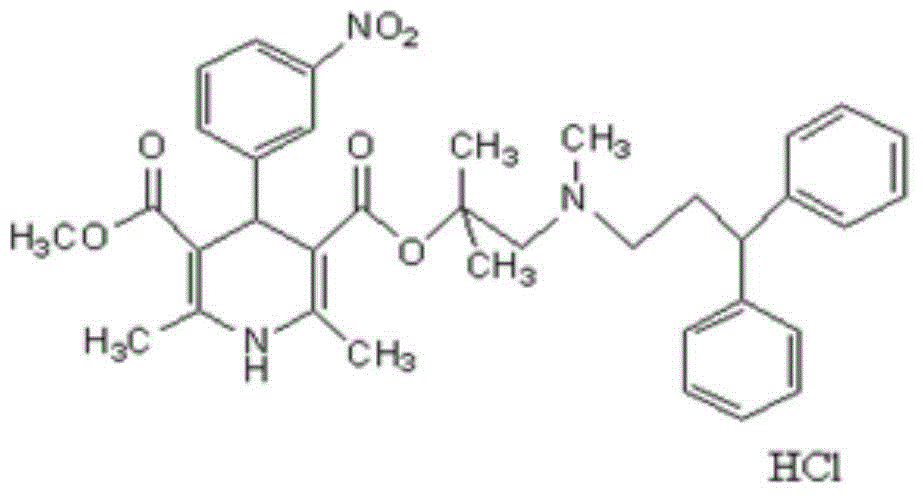
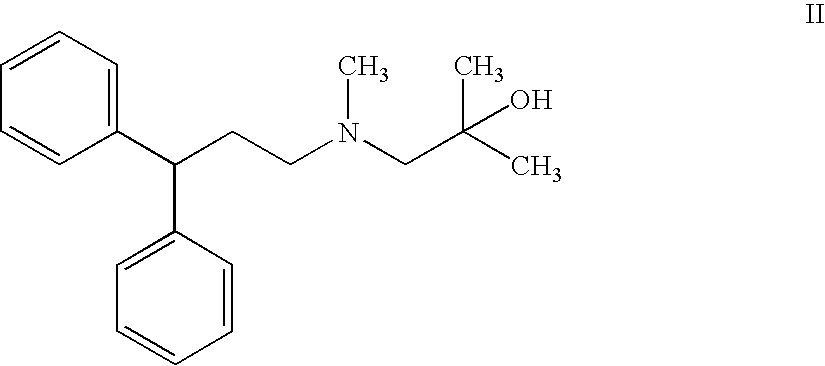
Crude and crystalline forms of lercanidipine hydrochloride
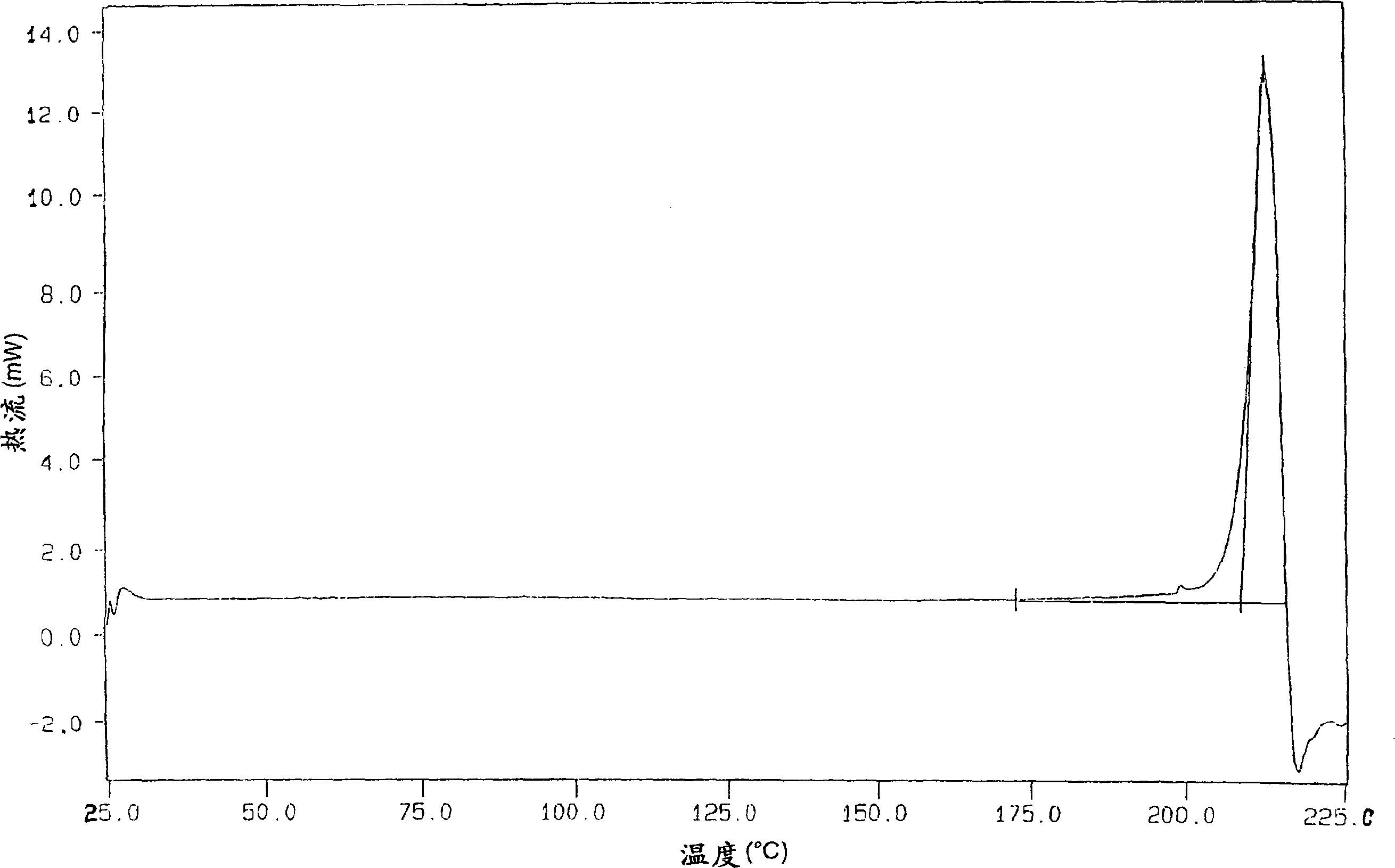
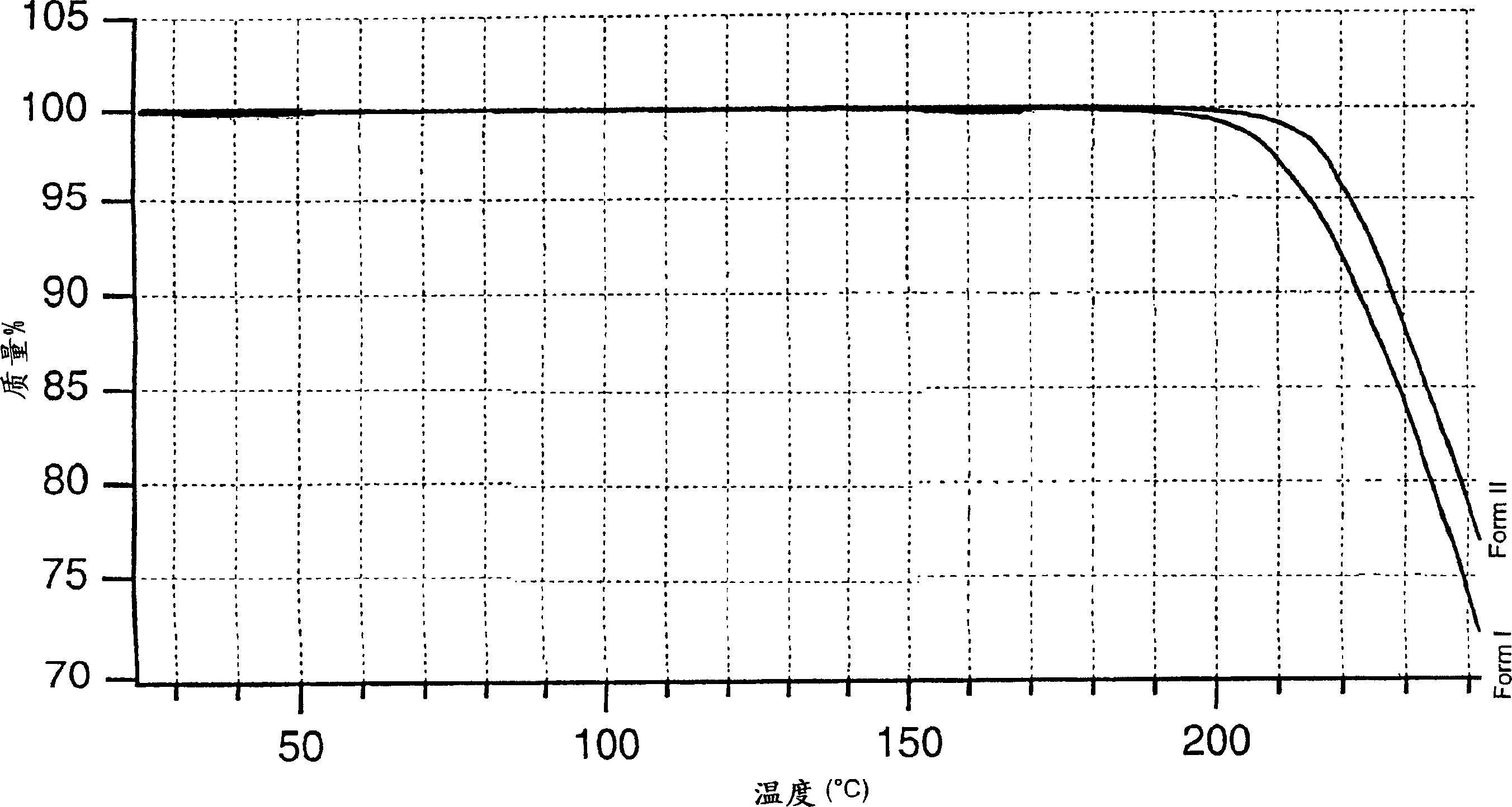
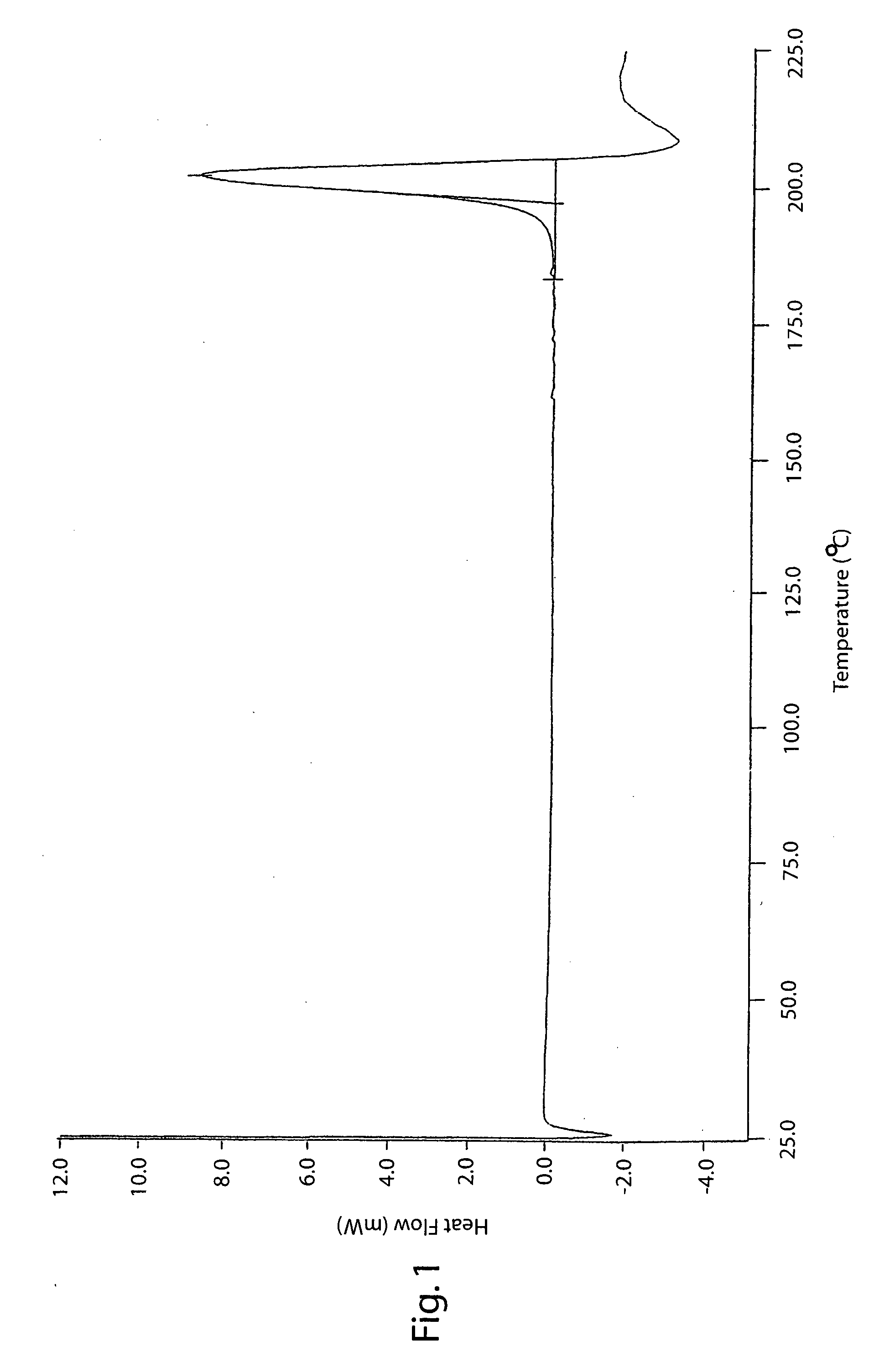
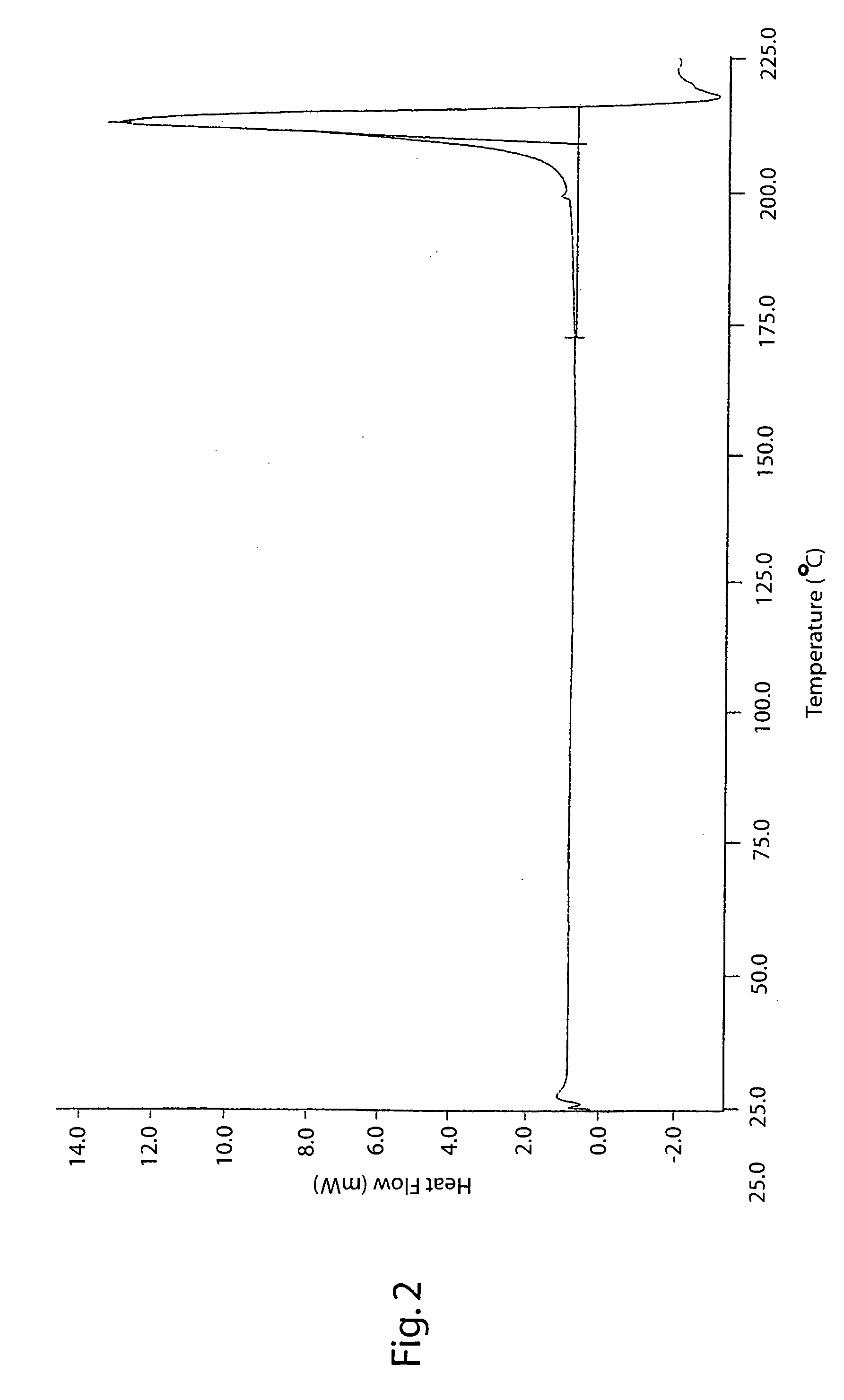
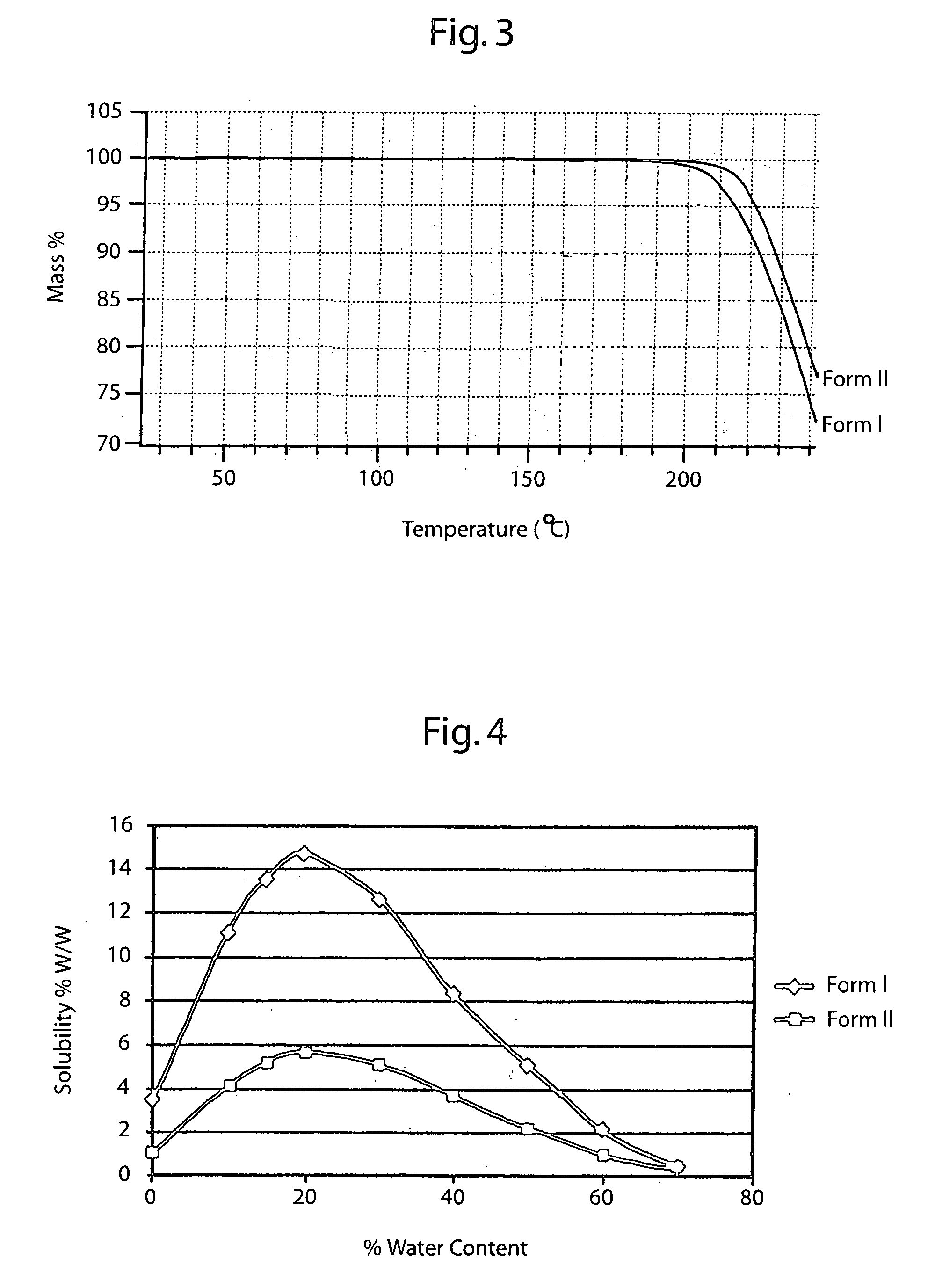
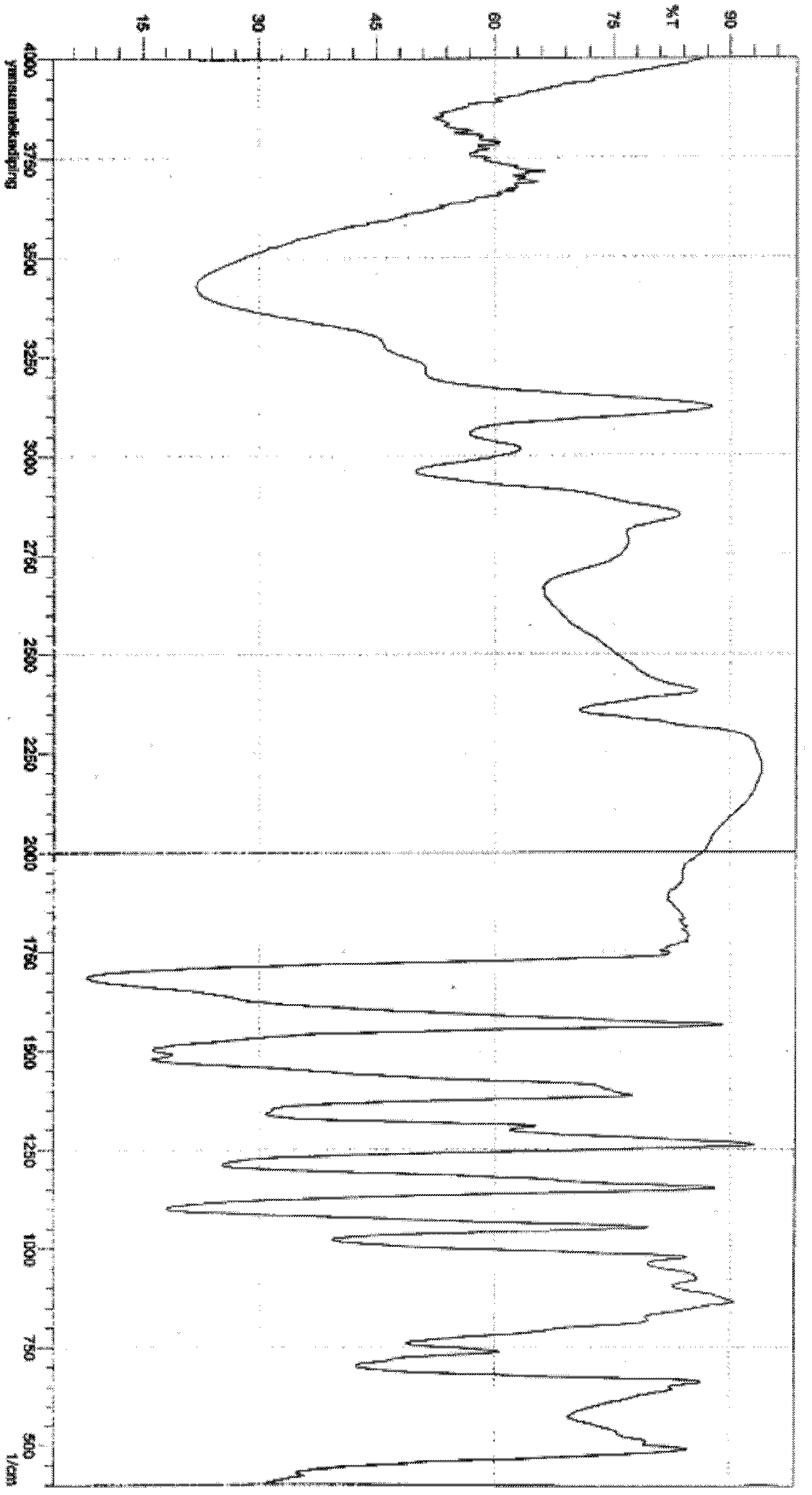
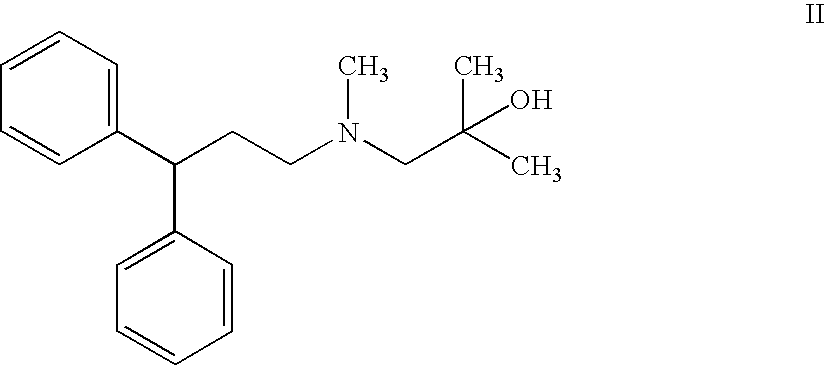
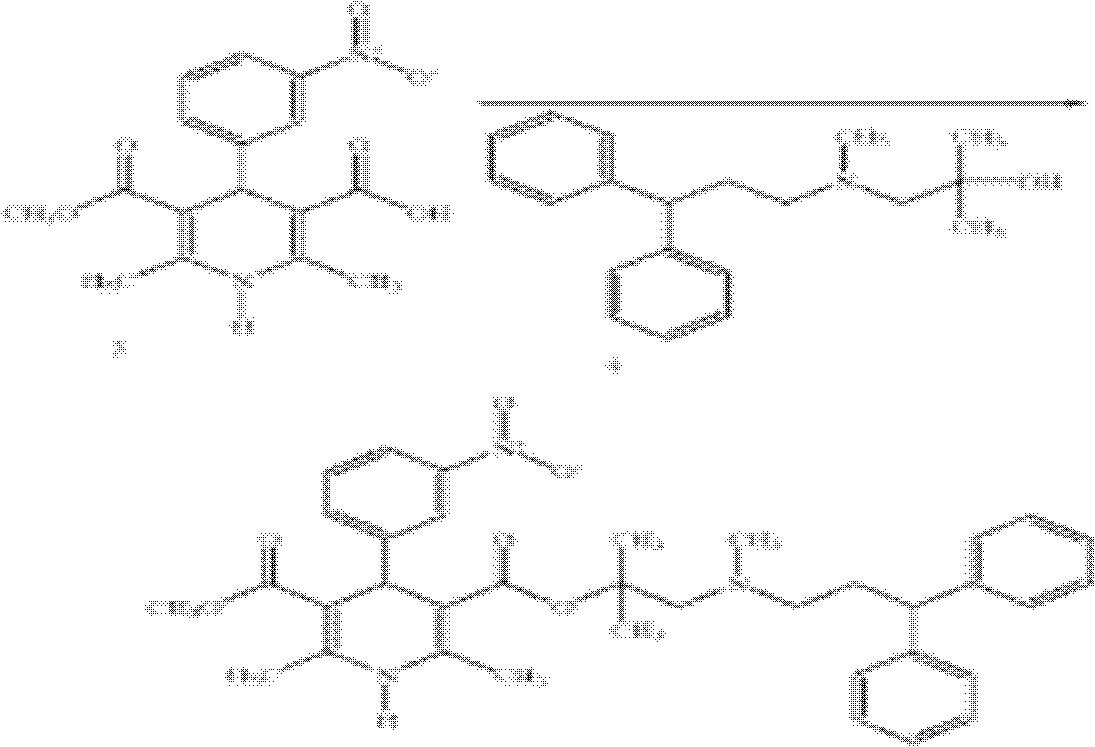
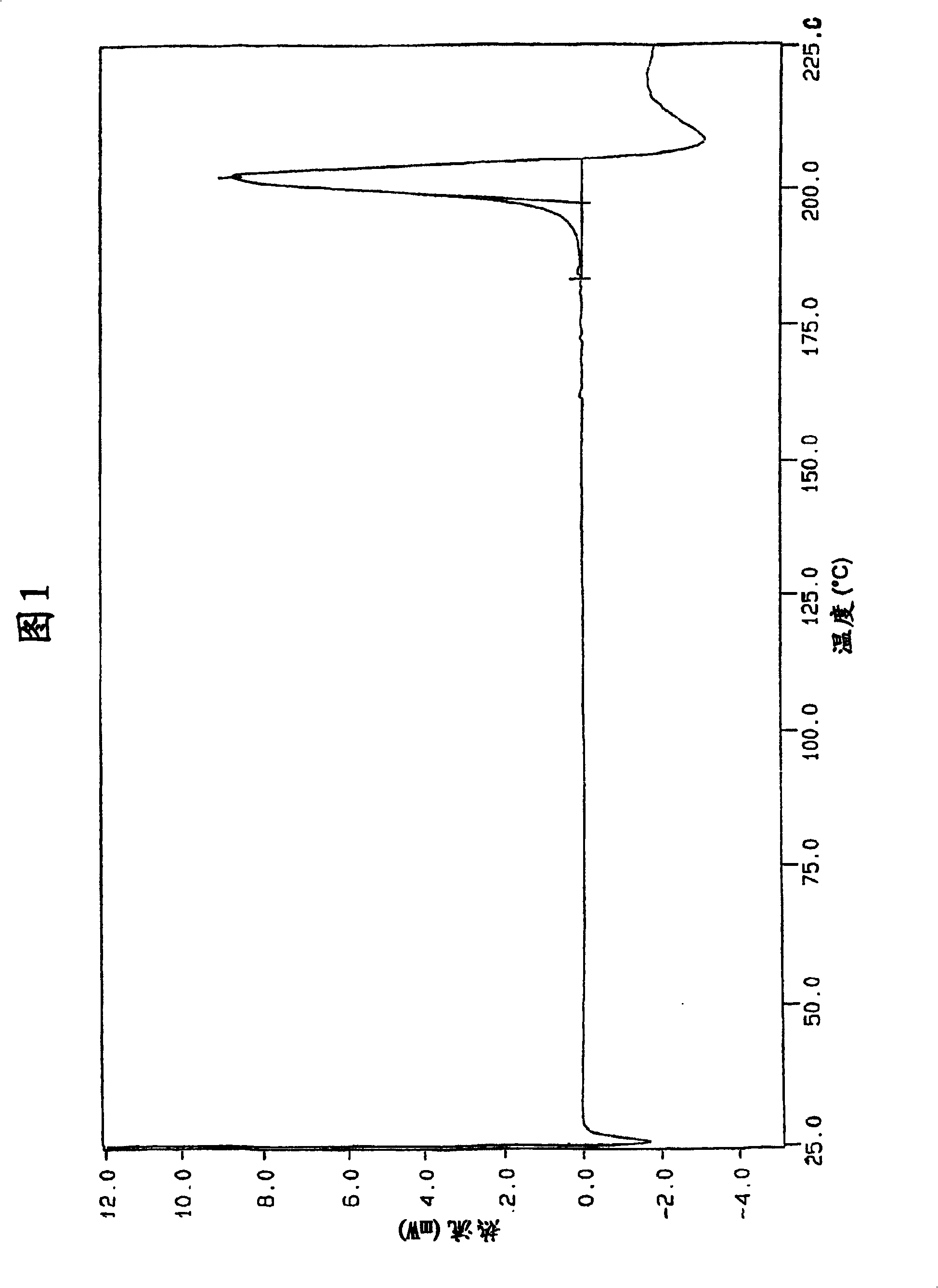
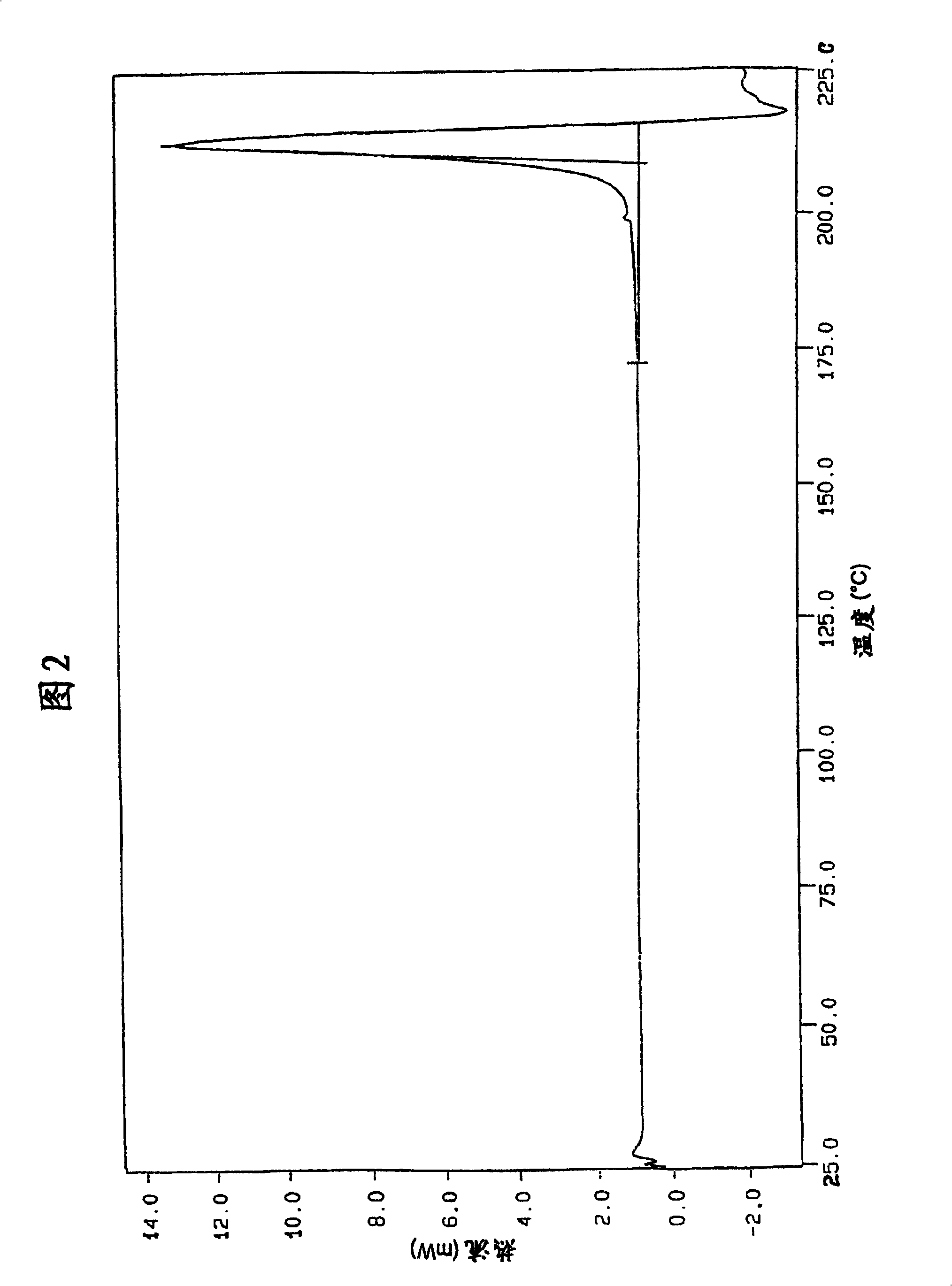
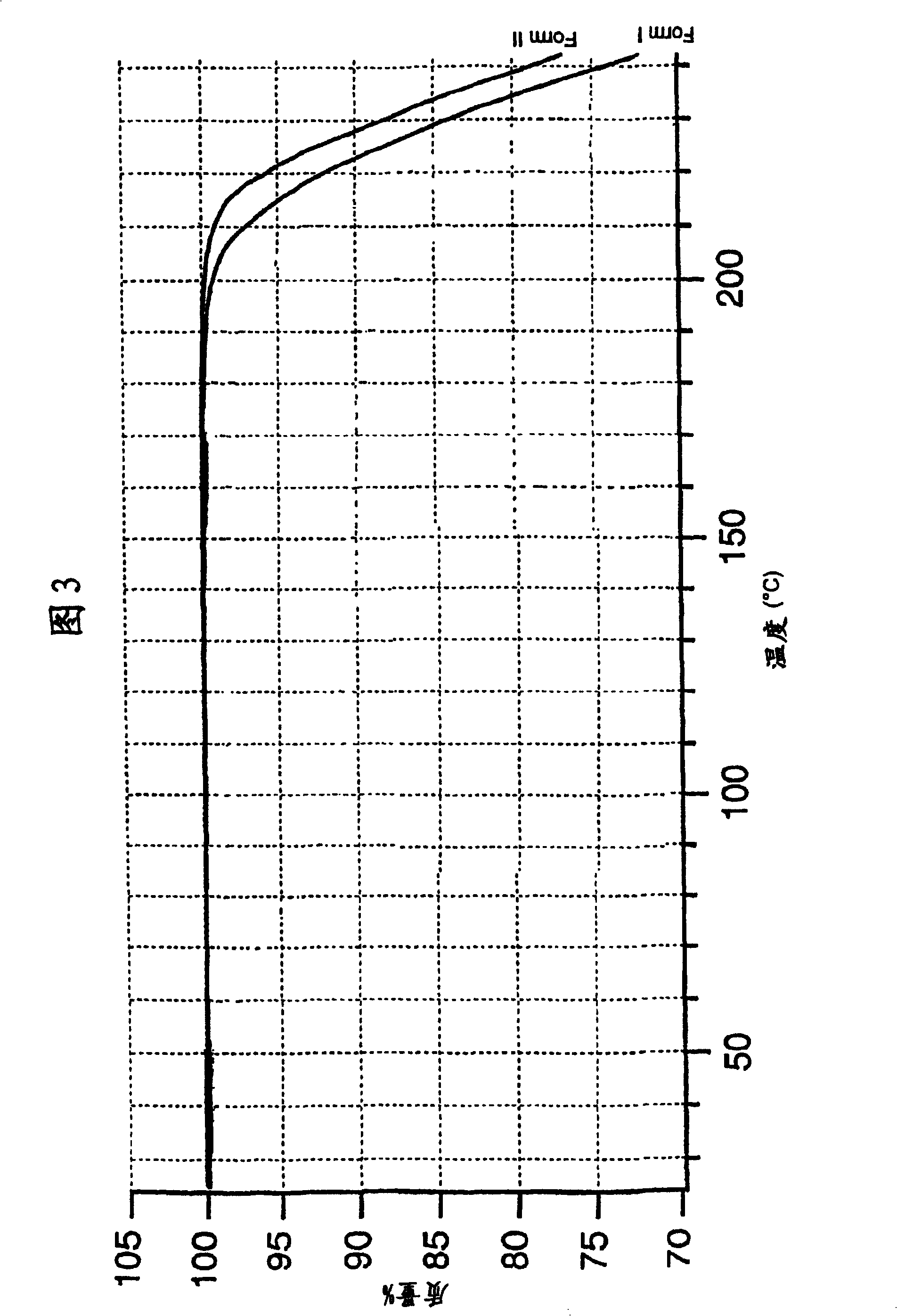
The invention describes novel lercanidipine crude Forms (A) and (B), novel lercanidipine hydrochloride crystalline Forms (I) and (II) obtained from said crude Forms, pharmaceutical, antihypertensive compositions containing as active agent at least one of the lercanidipine hydrochloride crystalline Forms (I) and (II) and methods of use thereof.
Owner:RECORDATIE IRELAND LTD
Novel crystalline polymorphic forms of lercanidipine hydrochloride and process for their preparation
InactiveCN1538957AOrganic chemistrySolution crystallizationMedicinal chemistryLercanidipine Hydrochloride
The invention is directed to novel crude forms and crystalline forms of lercanidipine hydrochloride, and to processes for the preparation of these forms. Pharmaceutical compositions comprising the novel crystalline forms also are contemplated.
Owner:RECORDATIE IRELAND LTD
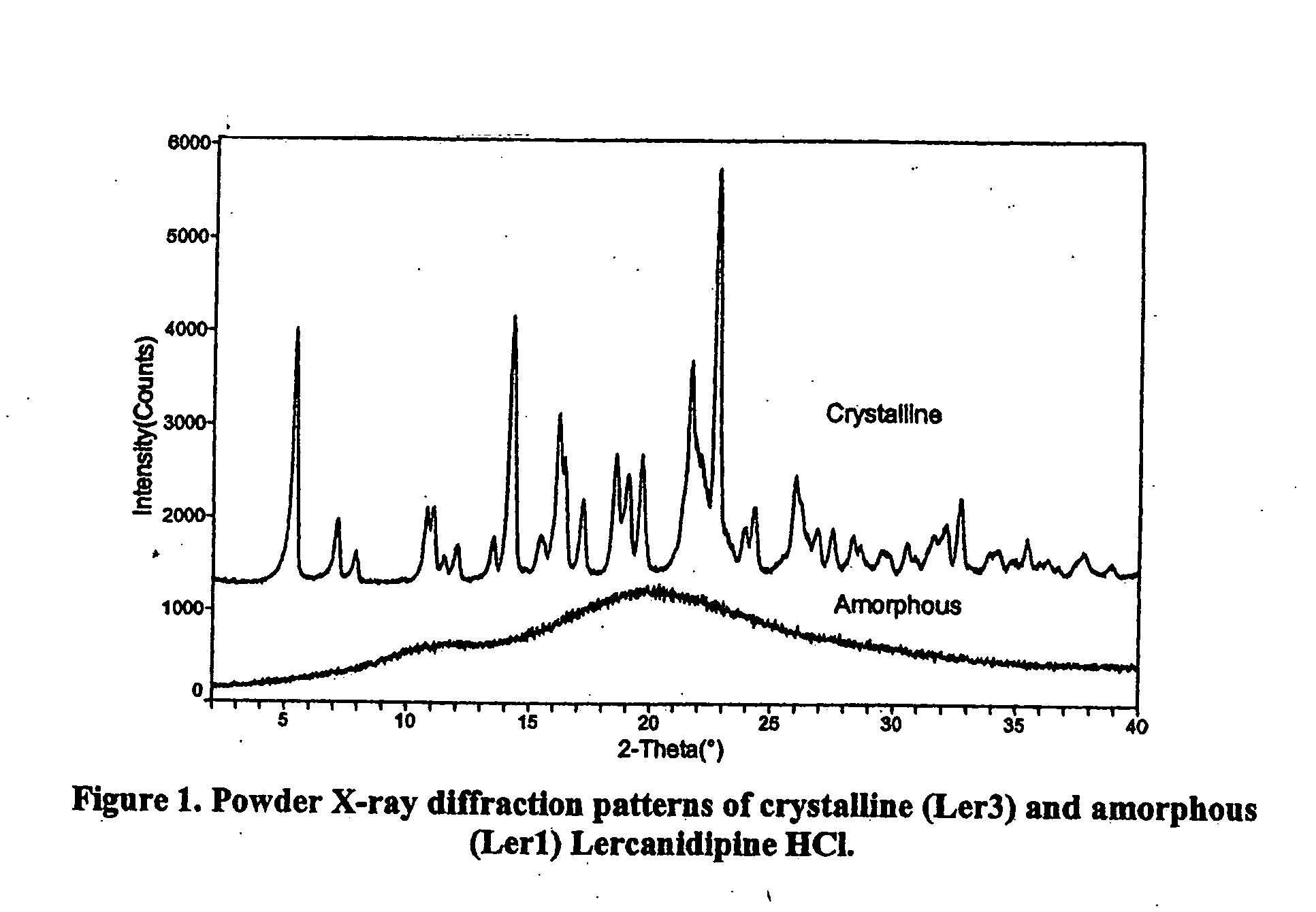
Amorphous lercanidipine hydrochloride and uses thereof
ActiveUS20060211742A1Good water solubilityRapid onsetBiocideOrganic compounds purification/separation/stabilisationPharmacologyLercanidipine Hydrochloride
The invention provides a substantially pure amorphous lercanidipine hydrochloride having a purity of at least 95% pure, preferably at least about 97% pure, more preferably at least about 99% pure, and still more preferably at least about 99.5% pure. The invention further relates to methods of preparing substantially pure amorphous lercanidipine, as well as methods of providing rapid relief from hypertension by administering the substantially pure amorphous lercanidipine hydrochloride of the present invention to a patient in need of such treatment.
Owner:RECORDATIE IRELAND LTD
Lercanidipine hydrochloride crystal and preparation method thereof
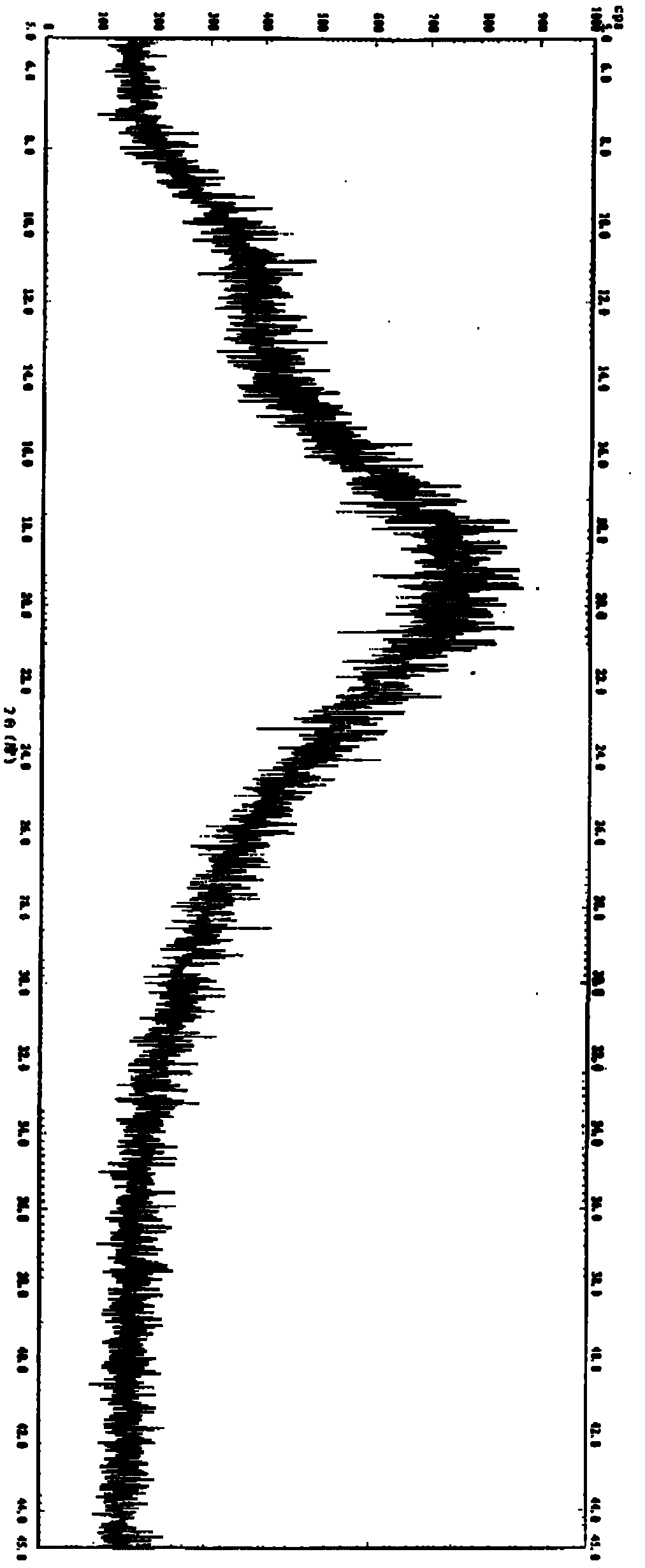
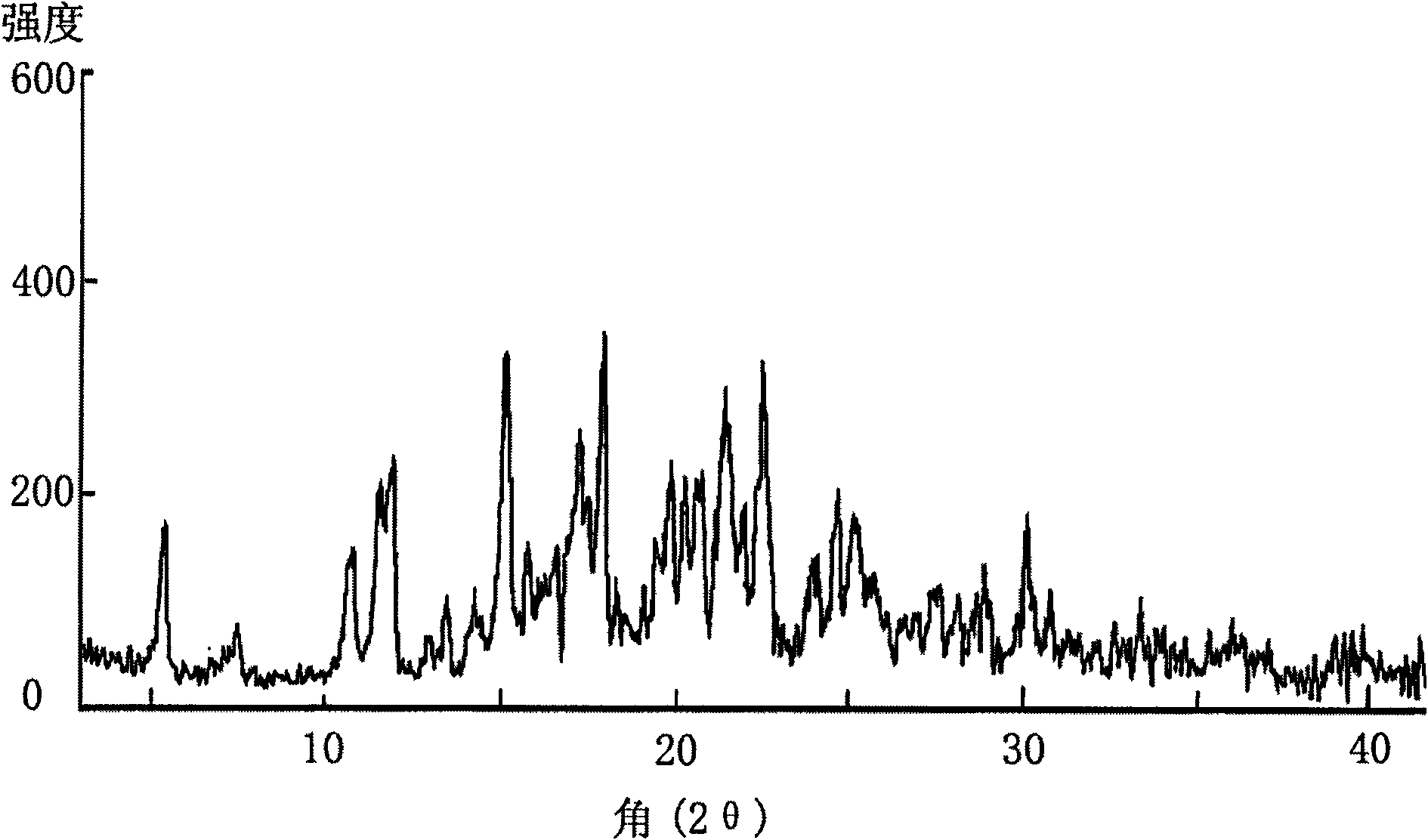
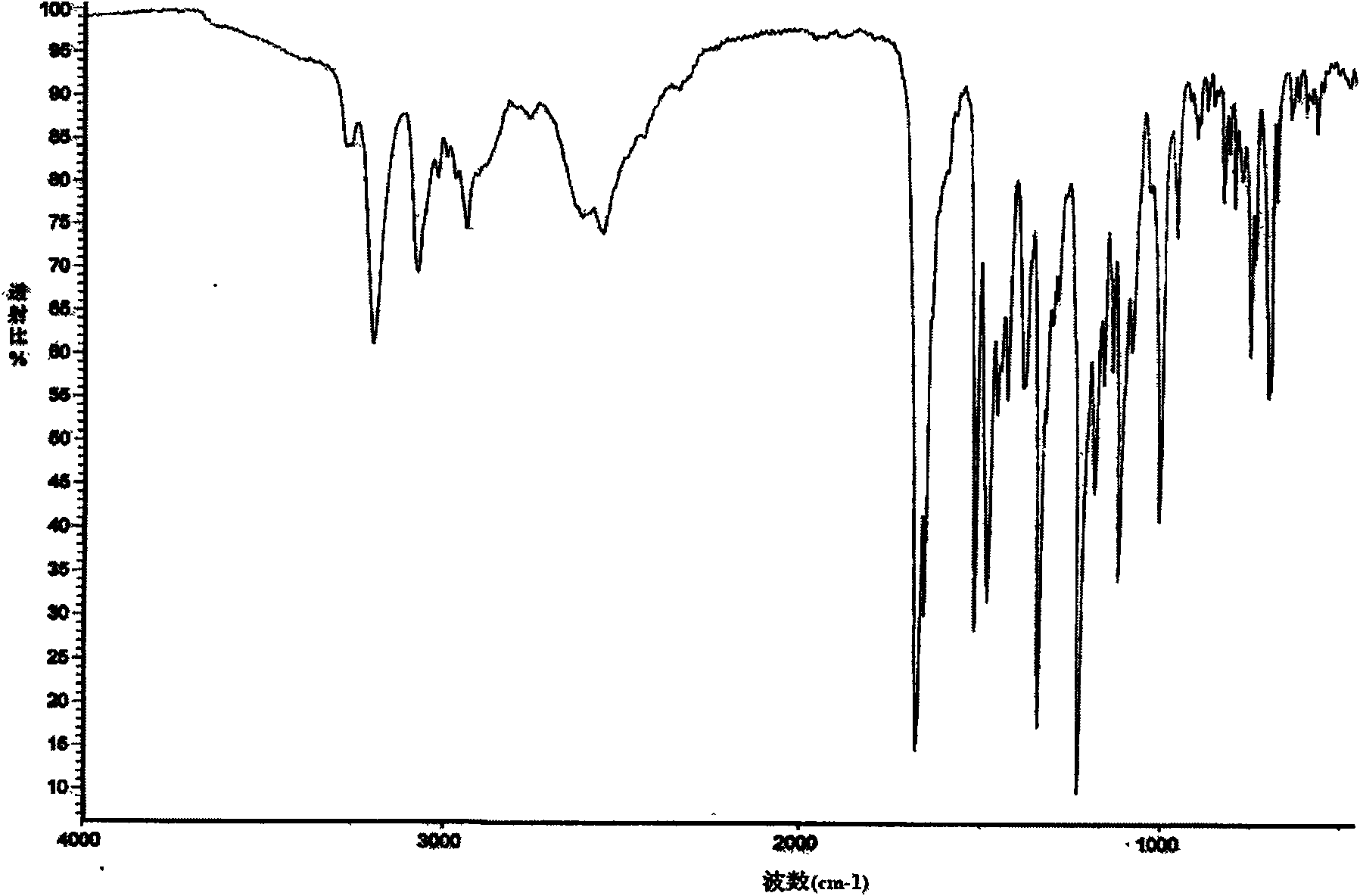
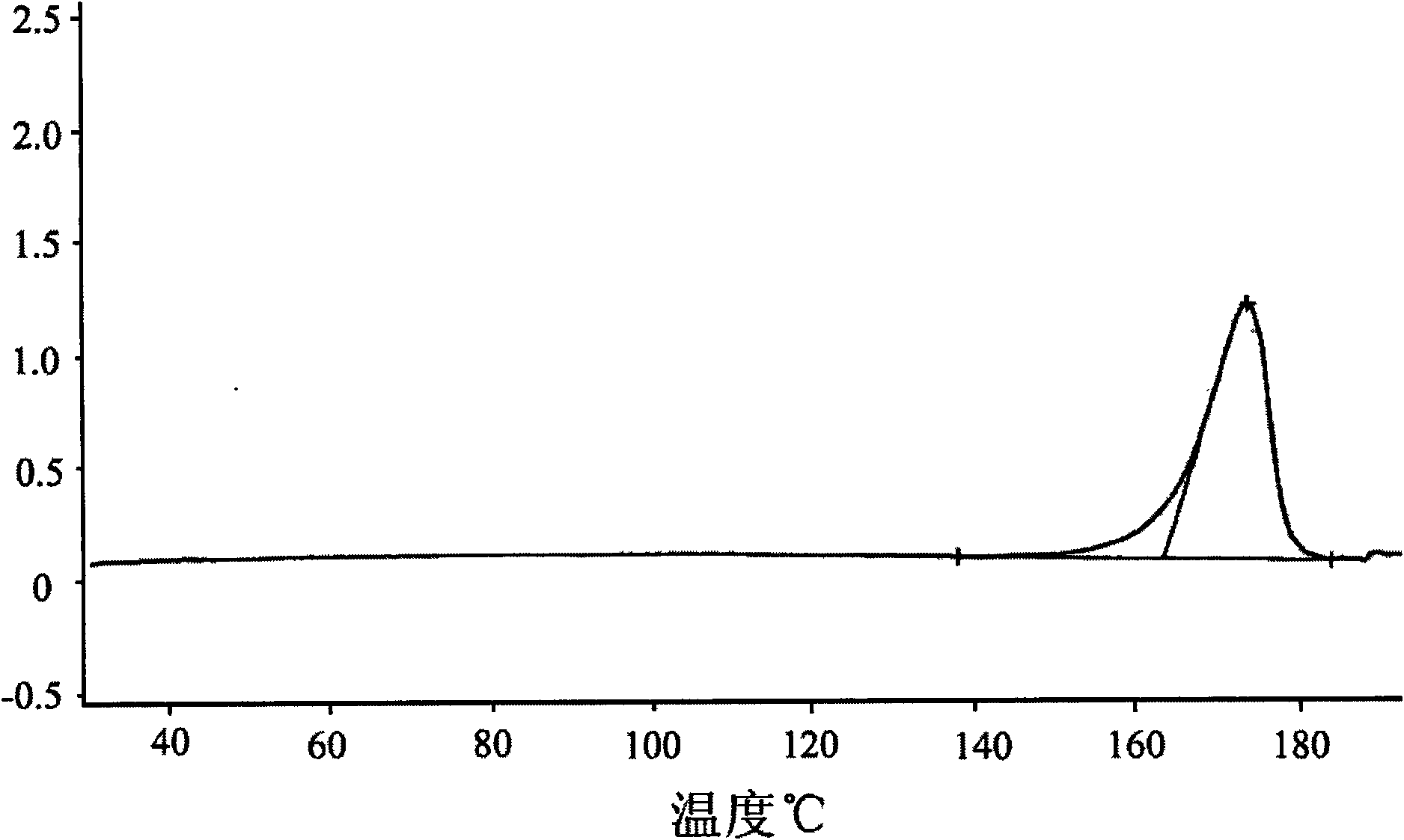
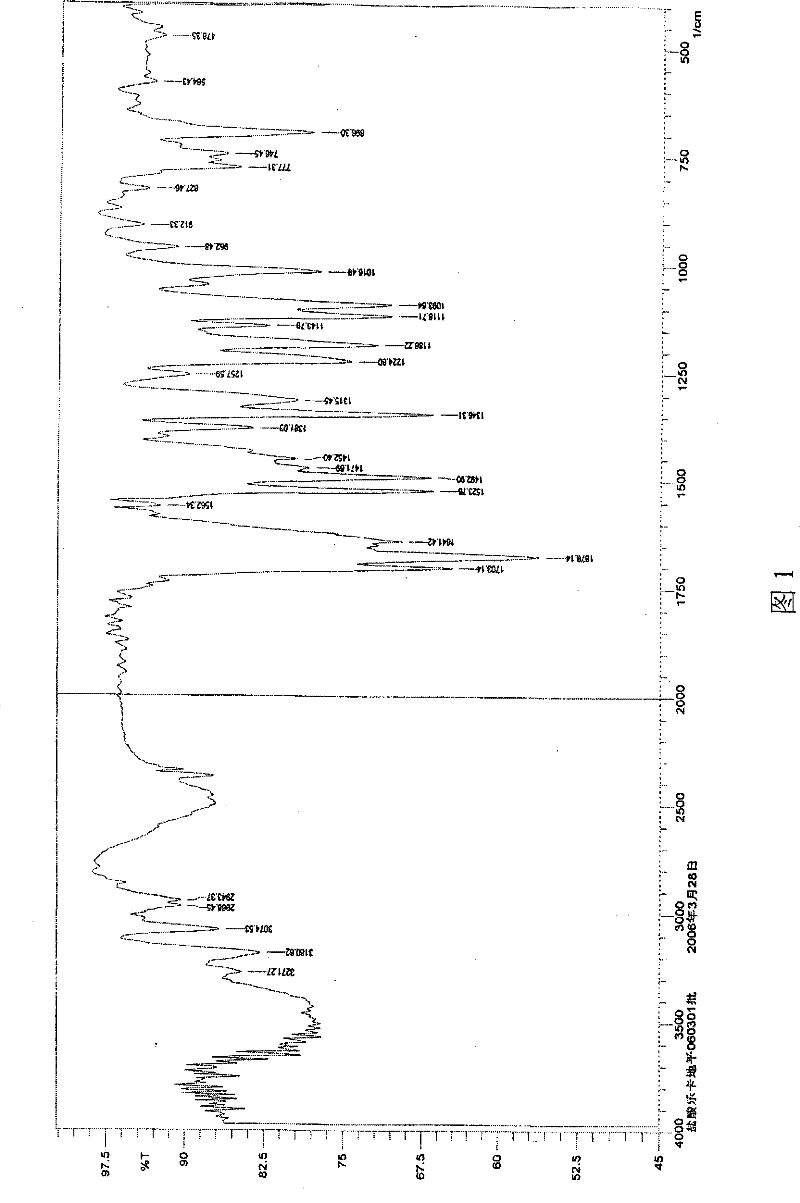
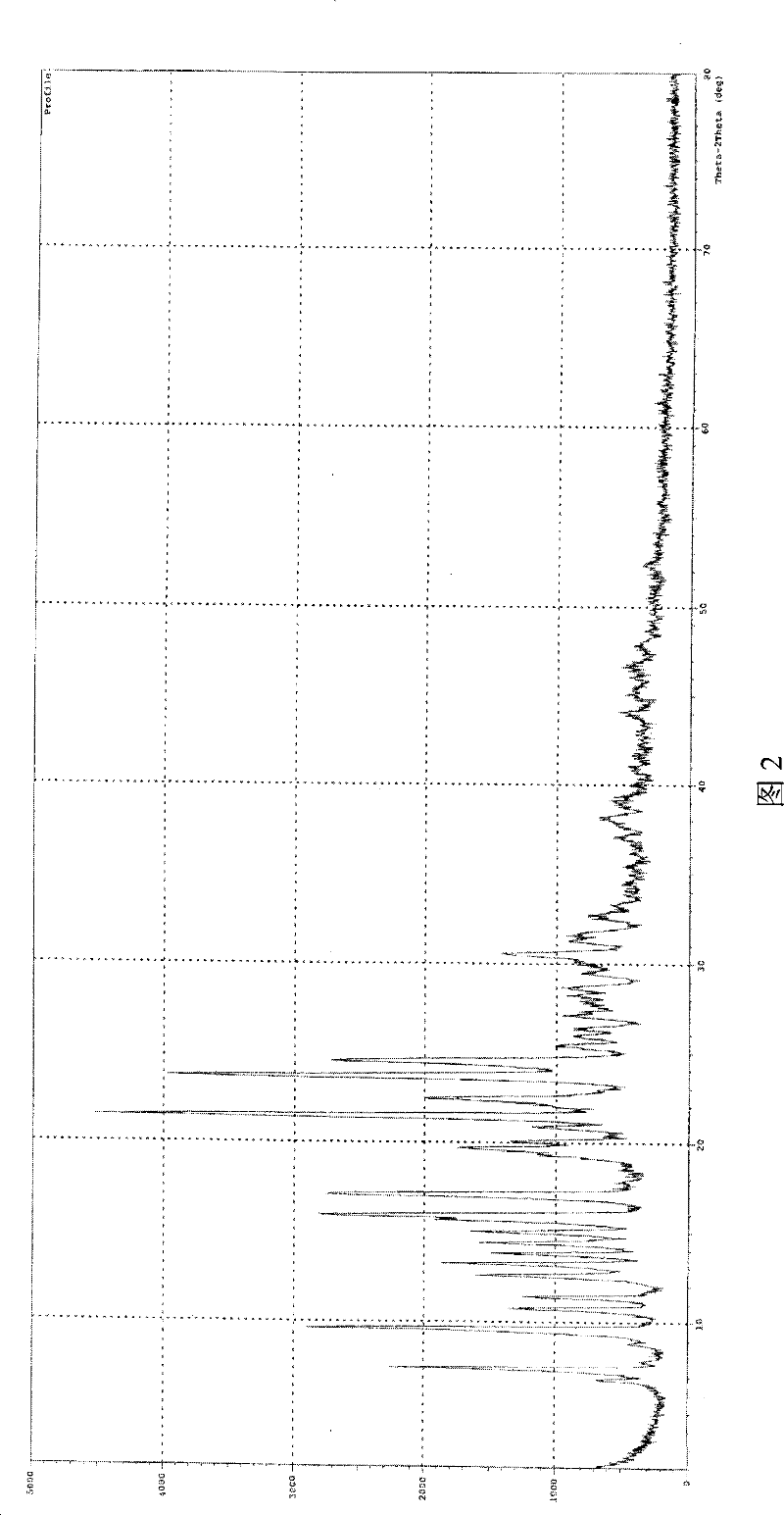
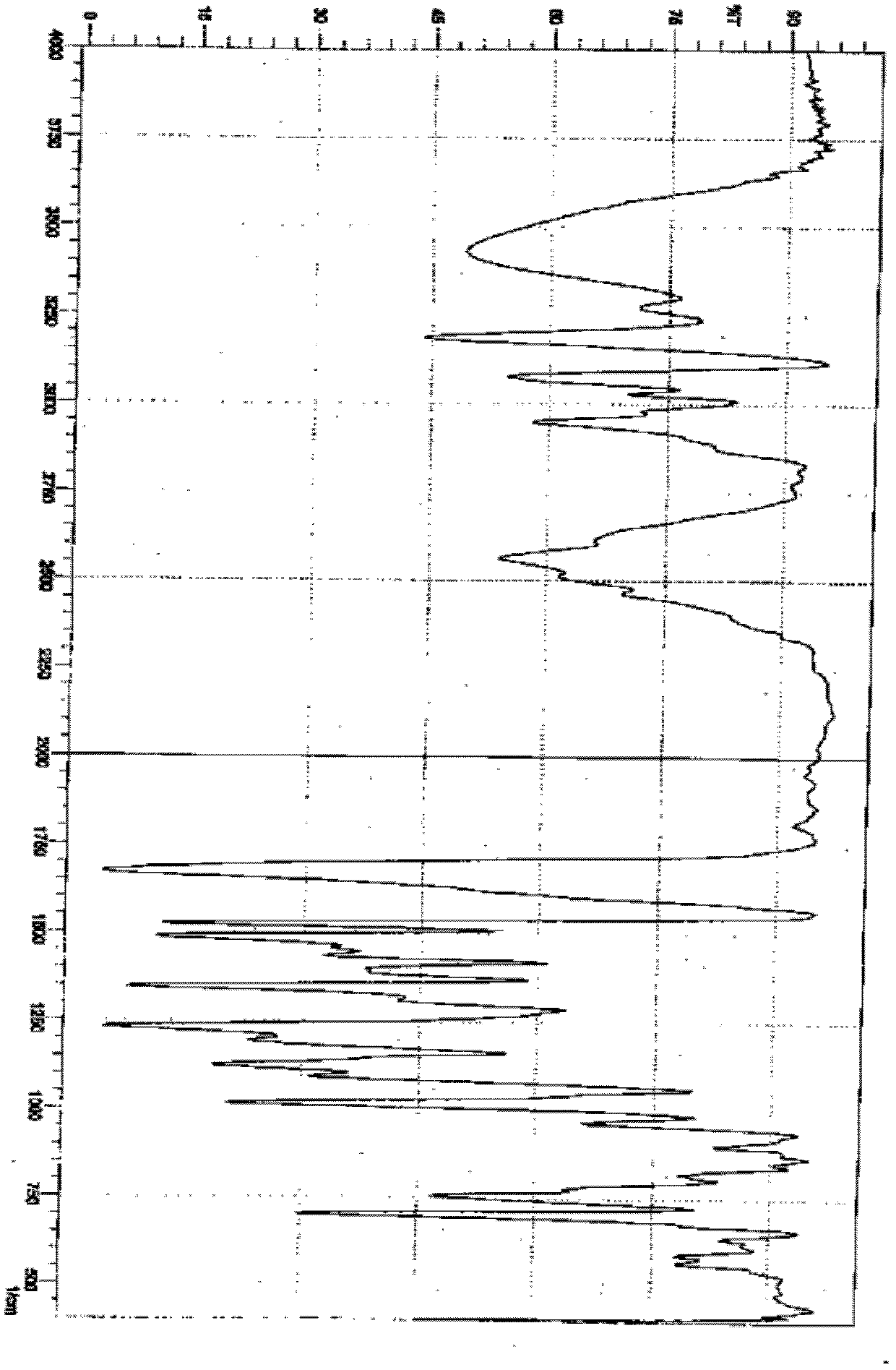
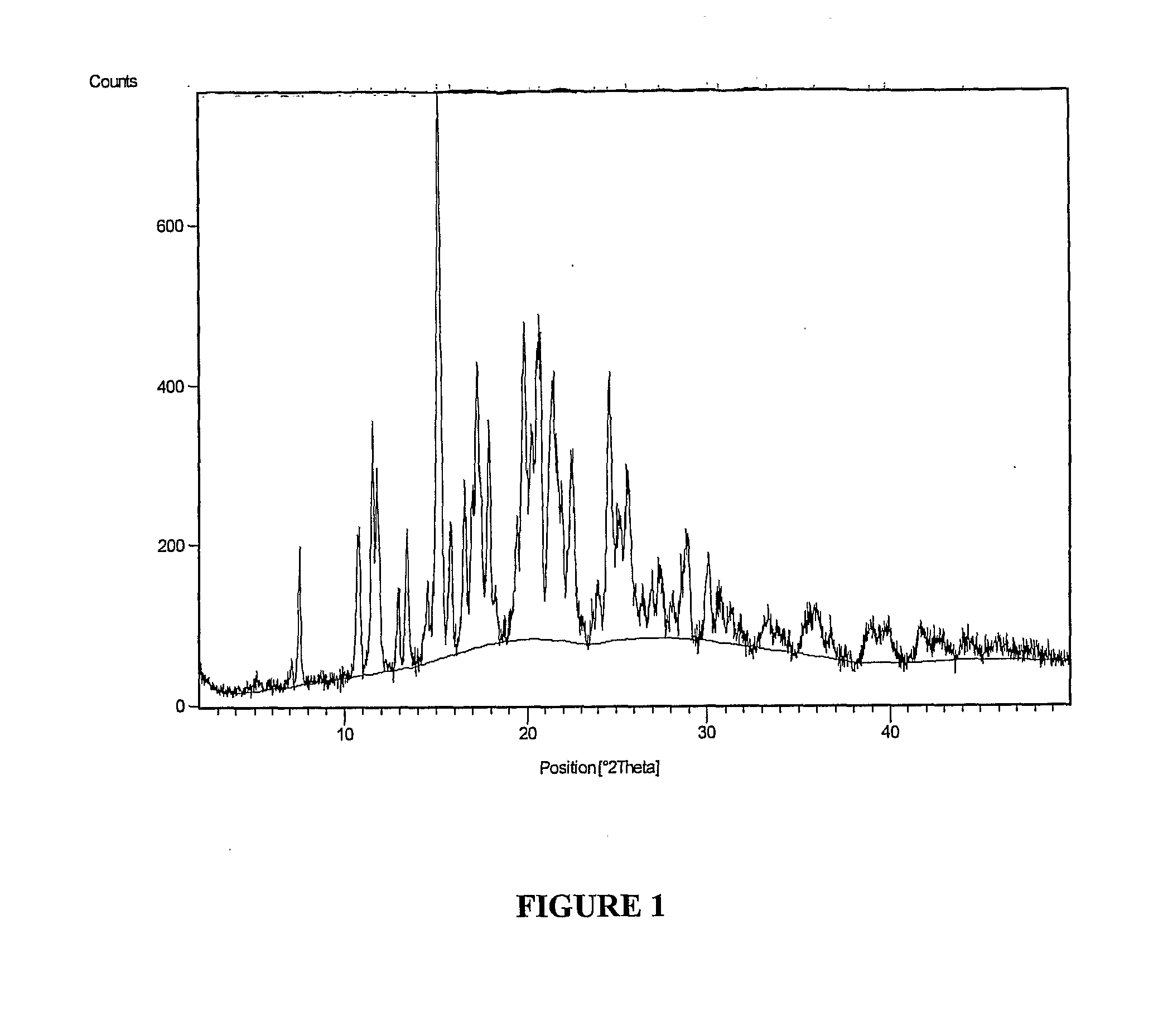
The invention is a lercandipine hydrochloride crystal (form (HX) crystal) and the preparation method. The crystal has no crystalline water and virgule or crystalline solvent, and is a novel high-purity stable crystalline form, which is more suitable for the large-scale industrial production. The invention is characterized in the simple and easy operation; thus the invention is conducive to the preparation of the drug combination and the clinical use. The crystal uses the Cu-K alpha radiation; the X-ray powder diffraction spectra show expressed in the 2-theta angle has the following characteristics (see the above formula).
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD
Lercanidipine hydrochloride polymorphs and an improved process for preparation of 1,1,N-trimethyl-N-(3,3-diphenylpropyl)-2-aminoethyl acetoacetate
InactiveCN101868442AReduce riskEasy to operateOrganic active ingredientsOrganic compound preparationEthyl esterMedicinal chemistry
Disclosed herein is an improved, commercially viable and industrially advantageous process for the preparation of substantially pure Lercanidipine intermediate, 1,1,N- trimethyl-N-(3,3-diphenylpropyl)-2-aminoethyl acetoacetate. The intermediate is useful for preparing Lercanidipine, or a pharmaceutically acceptable salt thereof, in high yield and purity. The present invention further provides a novel crystalline form of Lercanidipine hydrochloride and a process for its preparation. The present invention also provides a process for the preparation of amorphous form of Lercanidipine hydrochloride.
Owner:ACTAVIS GRP PTC EHF
Lercanidipine hydrochloride and losartan potassium compound preparation and preparation method thereof
ActiveCN102600146AReduce adverse reactionsImprove protectionOrganic active ingredientsMetabolism disorderTolerabilitySodium starch
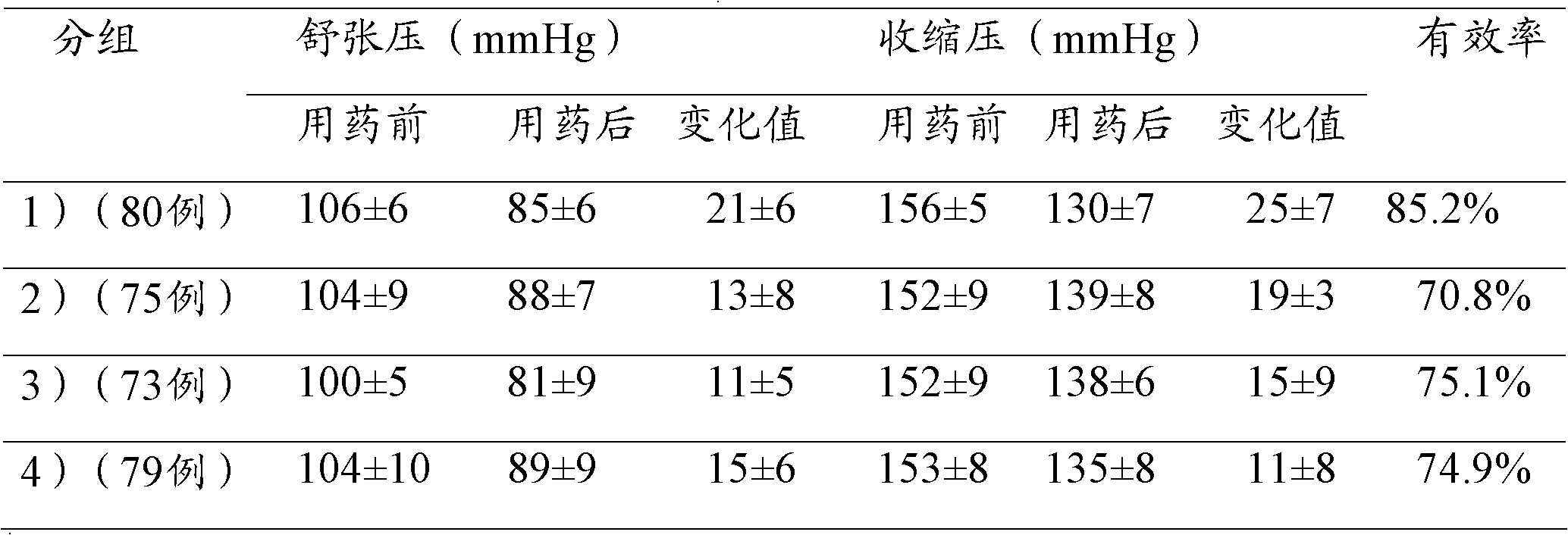
The invention relates to the field of medicines, and in particular discloses a lercanidipine hydrochloride and losartan potassium compound preparation. Particularly, the invention also provides a compound preparation which takes lercanidipine hydrochloride and losartan potassium as basic remedy, takes lactose monohydrate, microcrystalline cellulose, A-type sodium starch glycollate, povidone K30, magnesium stearate, pregelatinized starch and colloidal silicon dioxide as excipients, and takes white Opadry as a coating to prepare a tablet. Clinical tests prove that compared with the single-component preparation, the lercanidipine hydrochloride and losartan potassium compound preparation provided by the invention is remarkably increased in effective rate in light and moderate blood pressures, is remarkably reduced in occurrence rate of adverse effects, and has better clinical application prospect because patients have good tolerance.
Owner:ZHAOKE PHARMA HEFEI
Preparation method of lercanidipine
InactiveCN104744345ASimplify the manufacturing processIncrease productionOrganic chemistryCarboxylic acidSeed crystal
The invention relates to a preparation method of lercanidipine. The preparation method comprises the following steps of dissolving 2,6-dimethyl-5-methoxycarbonyl-4-(3-nitrophenyl)-1,4-dihydropyridyl-3-carboxylic acid in waterless dichloromethane and waterless dimethylformamide, dropwisely adding thionyl chloride into the solution under control of a temperature in a range of -4 to 1 DEG C, then carrying out stirring for 1h, dropwisely adding an anhydrous methylene chloride solution of 2,N-dimethyl-N-(3,3-dibenzylpropyl)-1-amino-2-ol into the mixed solution at a temperature of -10 to 0 DEG C, then carrying out stirring at a temperature of 0 DEG C for 3h, standing the mixture at a room temperature for 18-20h, carrying out washing orderly by saturated salt water, a sodium carbonate solution with a concentration of 10%, saturated salt water, hydrochloric acid with content of 1mol / L and saturated salt water, adding lercanidipine hydrochloride seed crystals into the washed product, carrying out standing at a temperature of 0-5 DEG C for 1d, carrying out filtration and carrying out anhydrous ethanol re-crystallization to obtain lercanidipine hydrochloride. The preparation method of lercanidipine has the advantages of simple preparation processes, high yield and low cost.
Owner:李磊
Novel crude and crystalline forms of lercanidipine hydrochloride
The invention describes novel lercanidipine crude Forms (A) and (B), novel lercanidipine hydrochloride crystalline Forms (I) and (II) obtained from said crude Forms, pharmaceutical, antihypertensive compositions containing as active agent at least one of the lercanidipine hydrochloride crystalline Forms (I) and (II) and methods of use thereof
Owner:RECORDATI IRELAND LTD
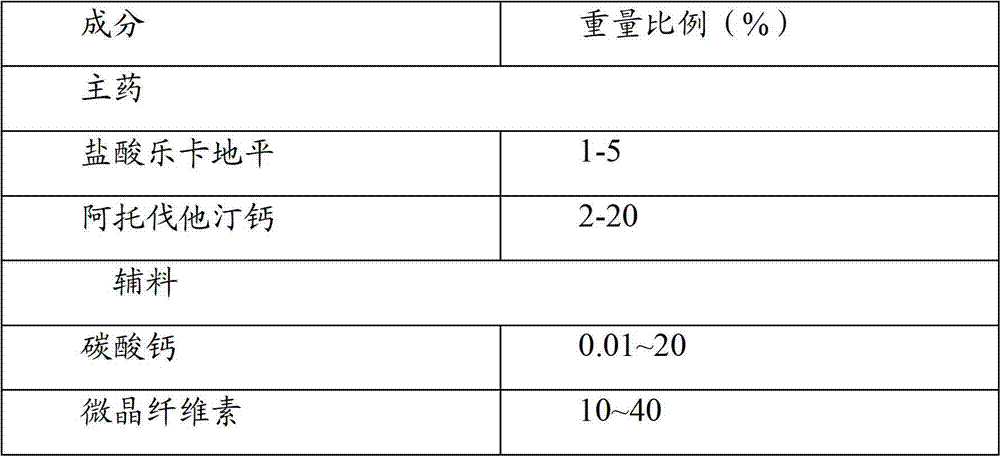
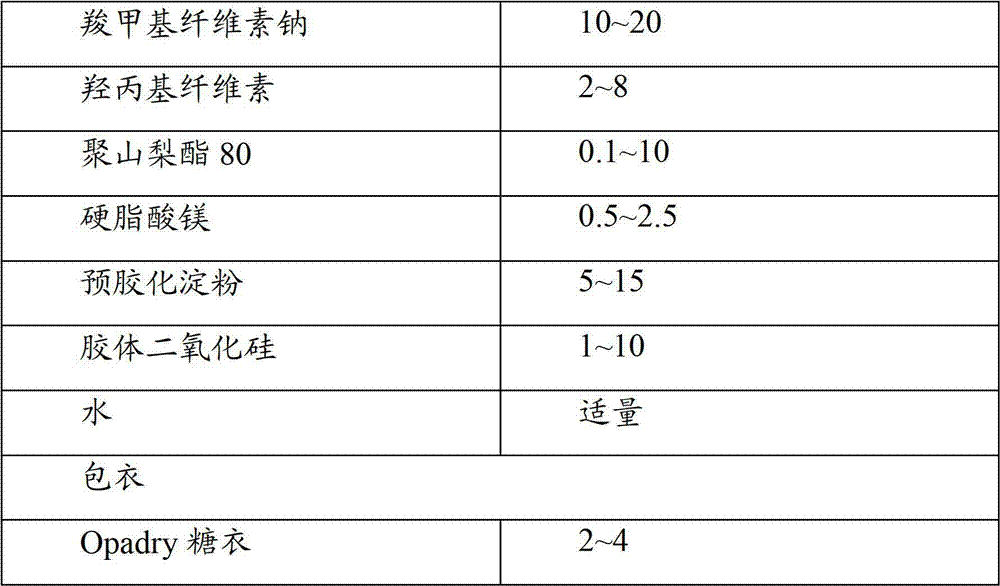
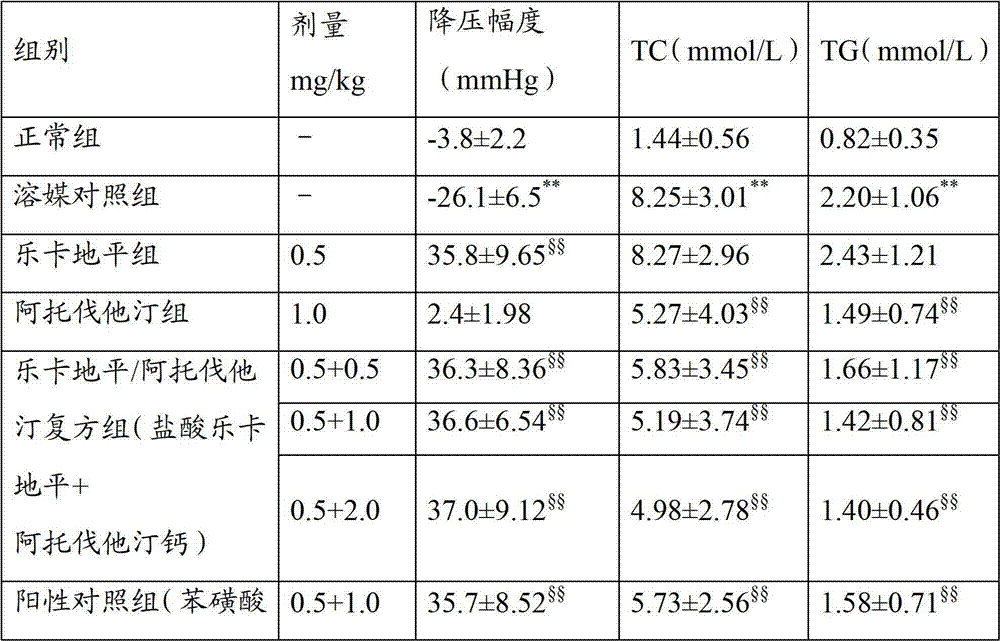
Compound preparation of lercanidipine and atorvastatin
ActiveCN102755322AImprove complianceMedication convenienceMetabolism disorderEster active ingredientsTolerabilityMagnesium stearate
The invention relates to the field of medicines and discloses a compound preparation of lercanidipine hydrochloride and atorvastatin calcium. The unit preparation comprises 5-20 mg of lercanidipine hydrochloride and 10-80 mg of atorvastatin calcium. The invention further provides another compound preparation which is prepared to tablets by taking lercanidipine hydrochloride and atorvastatin calcium as the main medicine, taking calcium carbonate, microcrystalline cellulose, sodium salt of carboxy methyl-cellulose, hydroxy propyl cellulose, polysorbate 80, magnesium stearate, amylum pregelatinisatum and colloidal silicon dioxide as auxiliary materials, and taking opadry as the coating. Clinical tests show that the compound preparation of lercanidipine and atorvastatin calcium plays a significant role in reducing pressure and fat, medicinal dose is reduced, incidence of untoward effects is reduced, toleration and medication compliance of patients are good, and the compound preparation has a good clinical application prospect.
Owner:ZHAOKE PHARMA GUANGZHOU
Lercanidipine hydrochloride tablets and preparation method thereof
ActiveCN105168165AHigh dissolution rateImprove yieldOrganic active ingredientsPharmaceutical non-active ingredientsCarboxymethyl starchIn vivo absorption
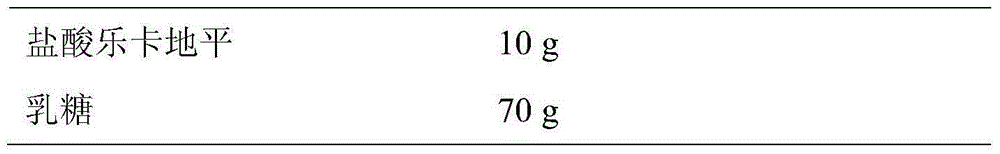
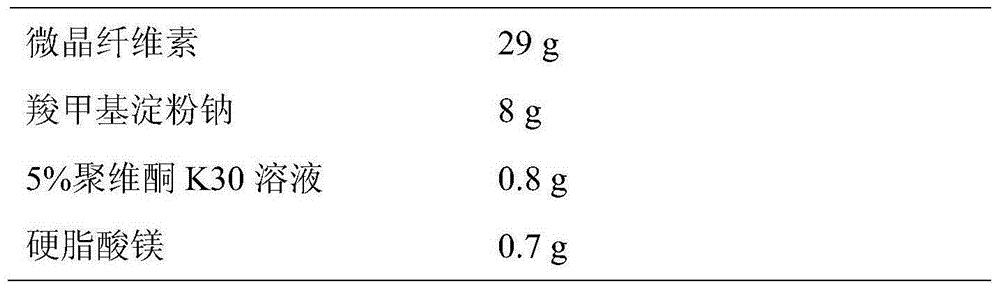
The invention discloses lercanidipine hydrochloride tablets. The lercanidipine hydrochloride tablets comprise lercanidipine hydrochloride, lactose, microcrystalline cellulose, carboxymethyl starch sodium, 5% povidone K30 solution and magnesium stearate, wherein the weight ratio of the lercanidipine hydrochloride, lactose, microcrystalline cellulose, carboxymethyl starch sodium, 5% povidone K30 solution and magnesium stearate is 10:70-75:29-33:8-12:0.8-1.2:0.7-3. The invention further provides a preparation method of the lercanidipine hydrochloride tablets. The method includes: the lercanidipine hydrochloride is mixed with the lactose and the like in an equal-quantity increasing manner and then evenly mixed with other auxiliary materials. The lercanidipine hydrochloride tablets have the advantages that the raw materials and auxiliary materials are evenly mixed, the tablets are even in lercanidipine hydrochloride content and high in dissolution rate, in vivo absorption is benefited, and the bioavailability of the tablets is increased.
Owner:JIANGSU FUBANG PHARMA
Crystal form of lercanidipine hydrochloride and preparation method thereof and crystal form-containing medicinal composition
ActiveCN102020602AHigh purityImprove stabilityOrganic active ingredientsOrganic chemistryX ray diffractogramLercanidipine Hydrochloride
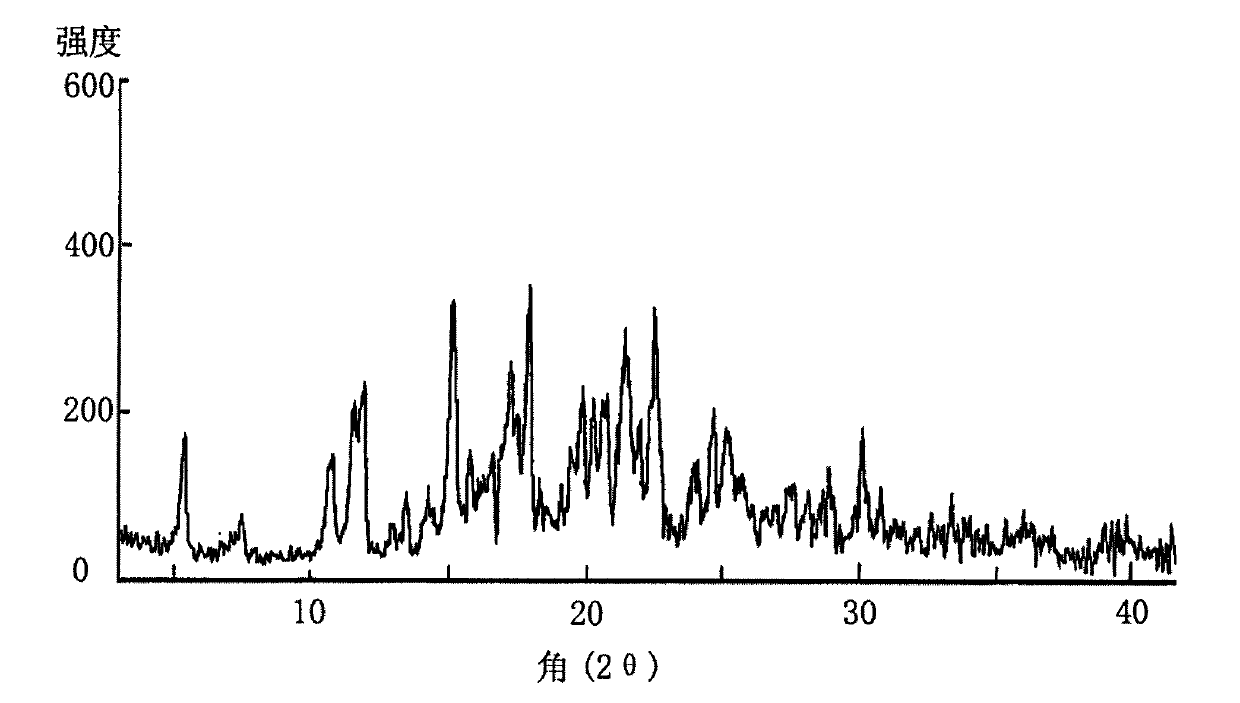
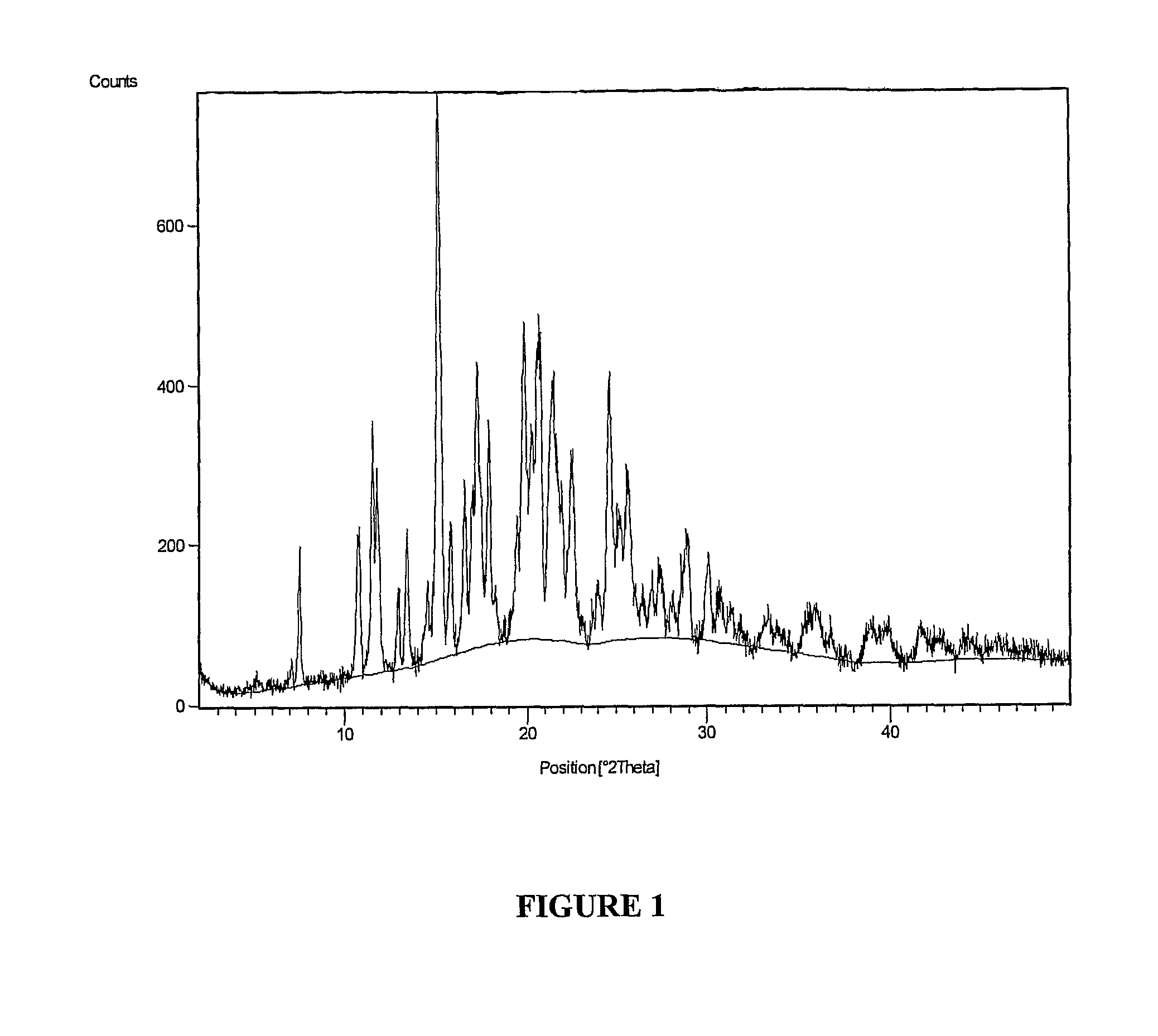
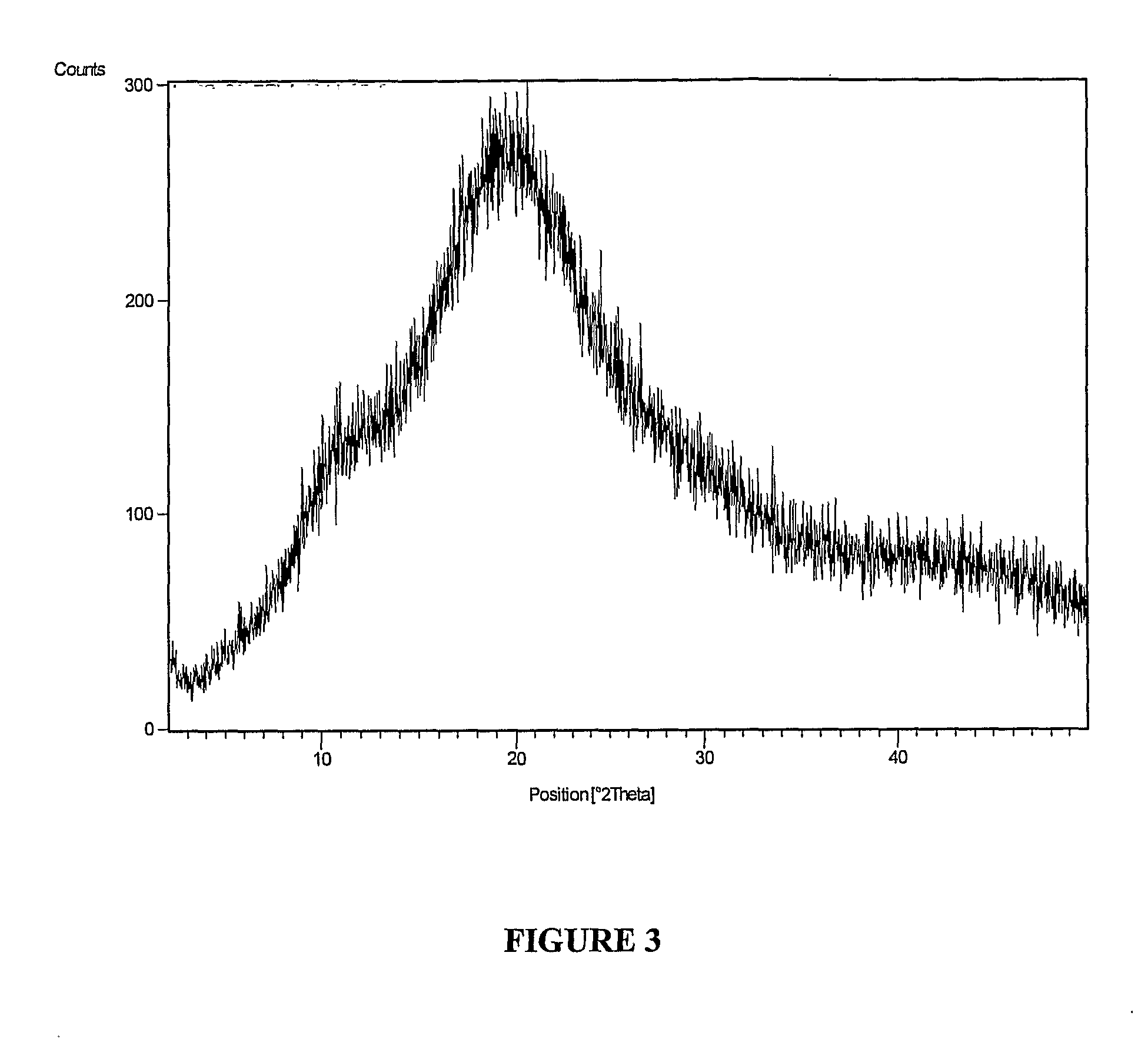
The invention provides a novel crystal form of lercanidipine hydrochloride, which has peaks at 5.38 DEG, 10.74 DEG, 11.54 DEG, 11.88 DEG, 15.14 DEG, 17.26 DEG, 17.92 DEG, 19.82 DEG, 20.22 DEG, 20.70 DEG, 21.44 DEG, 21.98 DEG and 22.56 DEG expressed by a 2theta angle in an X ray diffraction pattern. The novel crystal form has high purity (over 99.5 percent) and high stability, has low rigidity and is easy to crush after being dried, and facilitates preparing and using a medicinal composition. The invention also provides a preparation method for the crystal form and a crystal form-containing medicinal composition. Compared with the prior art, the preparation method has the advantages of simple process, mild preparation conditions and high yield.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
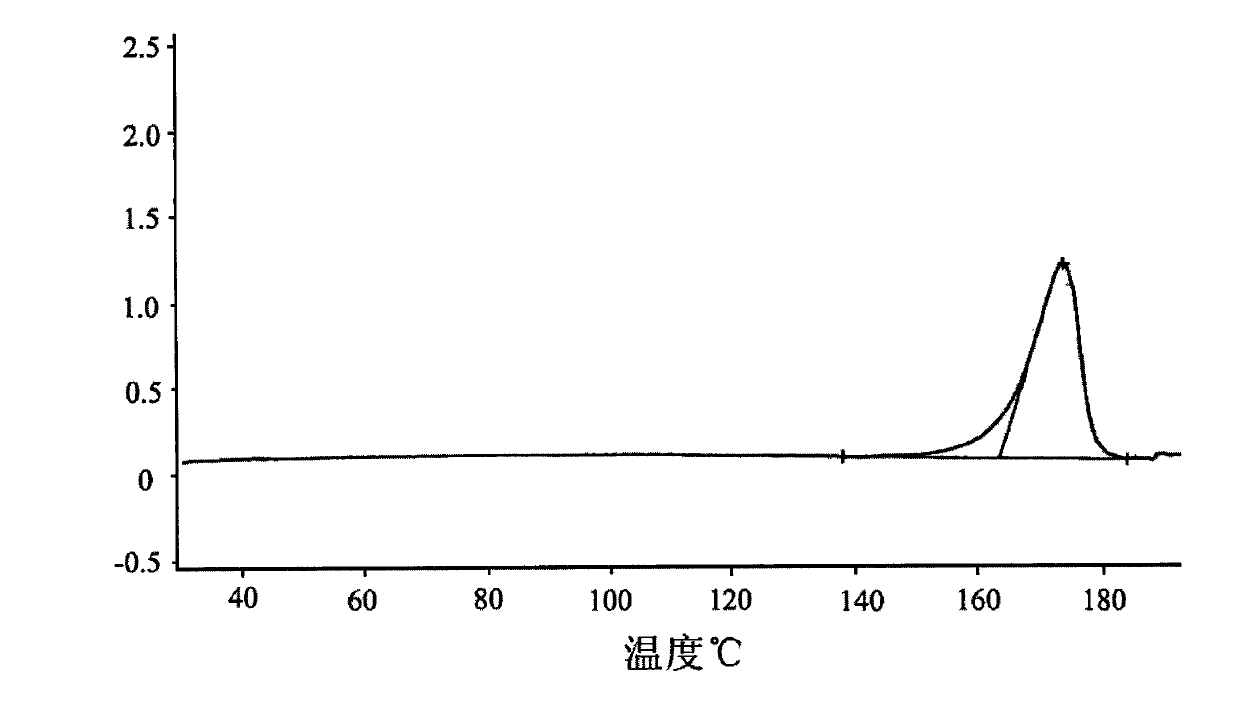
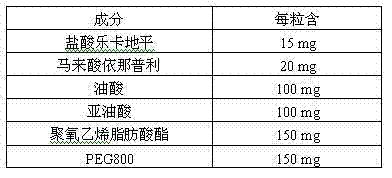
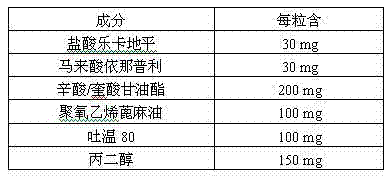
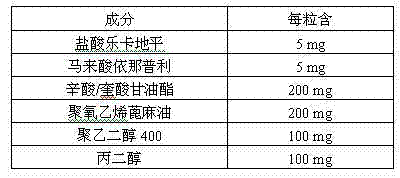
Pharmaceutical composition for treating hypertension
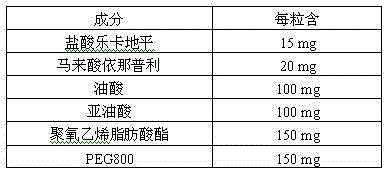
ActiveCN103239708AGood dispersionGood membrane permeabilityOrganic active ingredientsDipeptide ingredientsSolubilityHard Capsule
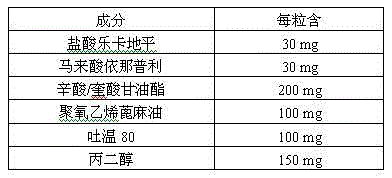
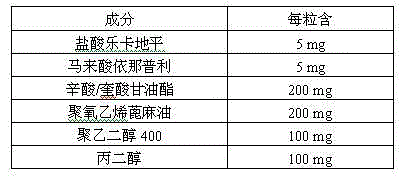
The invention provides a pharmaceutical composition for treating hypertension. The invention relates to a pharmaceutical composition comprising therapeutic doses of lercanidipine hydrochloride and enalapril maleate, and a proper amount of oil, a surfactant and an assisted surfactant. Preparative dosage forms particularly hard capsules and soft capsules are provided. The pharmaceutical composition increases solubility of a poorly soluble drug of lercanidipine hydrochloride and stability of enalapril maleate, and is suitable for the treatment of hypertension.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +1
HPLC (High Performance Liquid Chromatography) method for determining dissolution rate of Lercanidipine hydrochloride tablet
InactiveCN104807898AQuality improvementOvercoming the flaws of interferenceComponent separationHplc methodColumn temperature
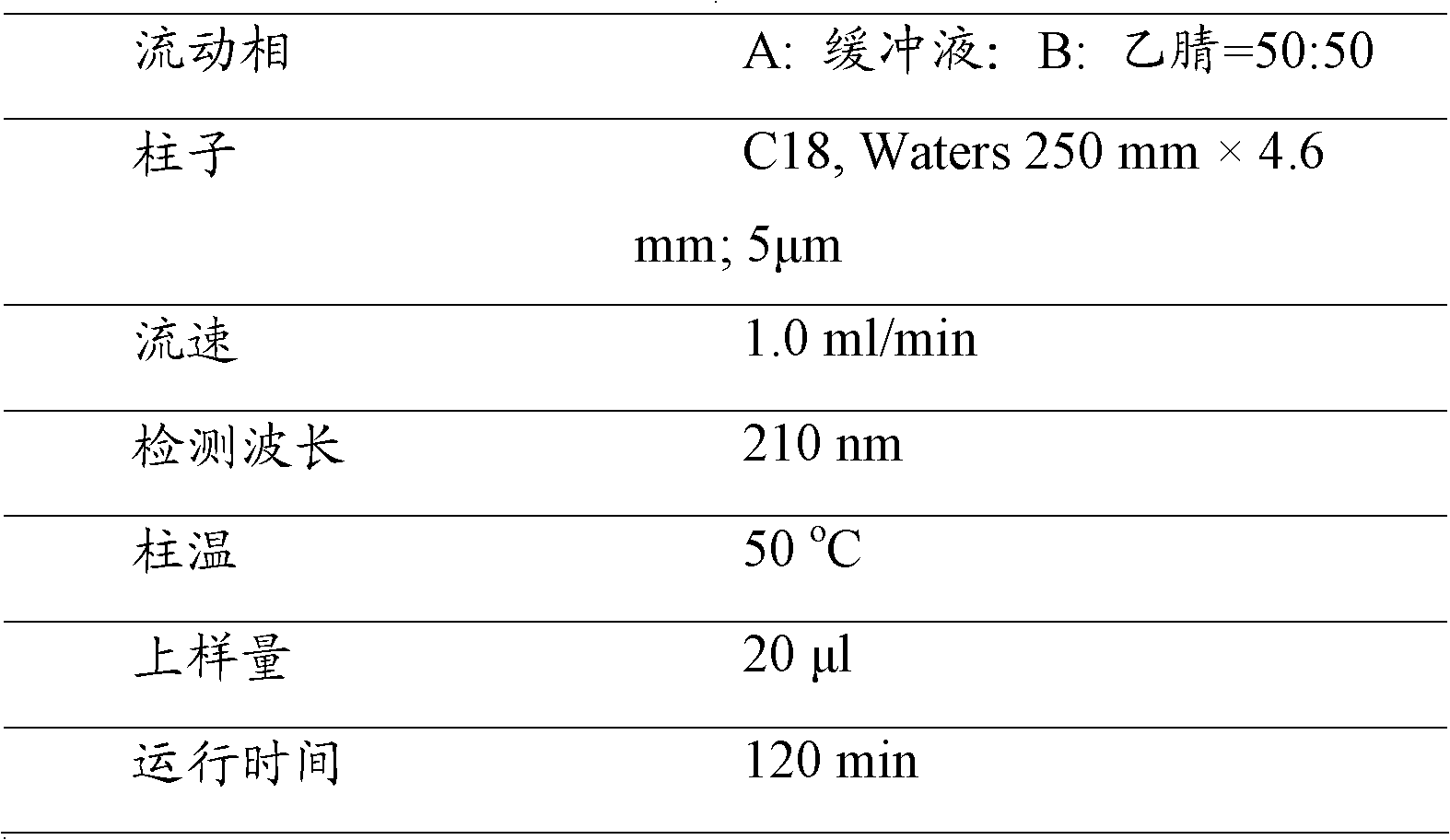
The invention relates to an HPLC (High Performance Liquid Chromatography) method for determining the dissolution rate of a Lercanidipine hydrochloride tablet. The method comprises the following steps: (1) test of the dissolution rate of the Lercanidipine hydrochloride tablet and preparation of a sample solution; (2) preparation of a reference solution of Lercanidipine hydrochloride; (3) HPLC determination, to be specific, performing determination analysis on the reference solution and the sample solution under the following chromatographic conditions: a chromatographic column is Waters SunFire C18 (4.6 mm*150 mm, 5 [mu]m), a flowing phase is formed by a 0.15 mol.L<-1> sodium perchlorate solution (of which the pH is adjusted by a 70% perchloric acid to be 3.0-4.0) and acetonitrile, the ratio of the sodium perchlorate solution to the acetonitrile is 40:60, the column temperature is 25-30 DEG C, the detection wavelength is 240 nm, the flow speed is 1.0 ml.min<-1>, and the sample injection amount is 40 [mu]L. A methodological test shows that the method is high in specificity, accurate in result, good in stability, simple and convenient to operate, and suitable for the determination of the dissolution rate of the Lercanidipine hydrochloride tablet, and can control the quality of the Lercanidipine hydrochloride tablet more scientifically and effectively.
Owner:CHONGQING MEDICAL UNIVERSITY
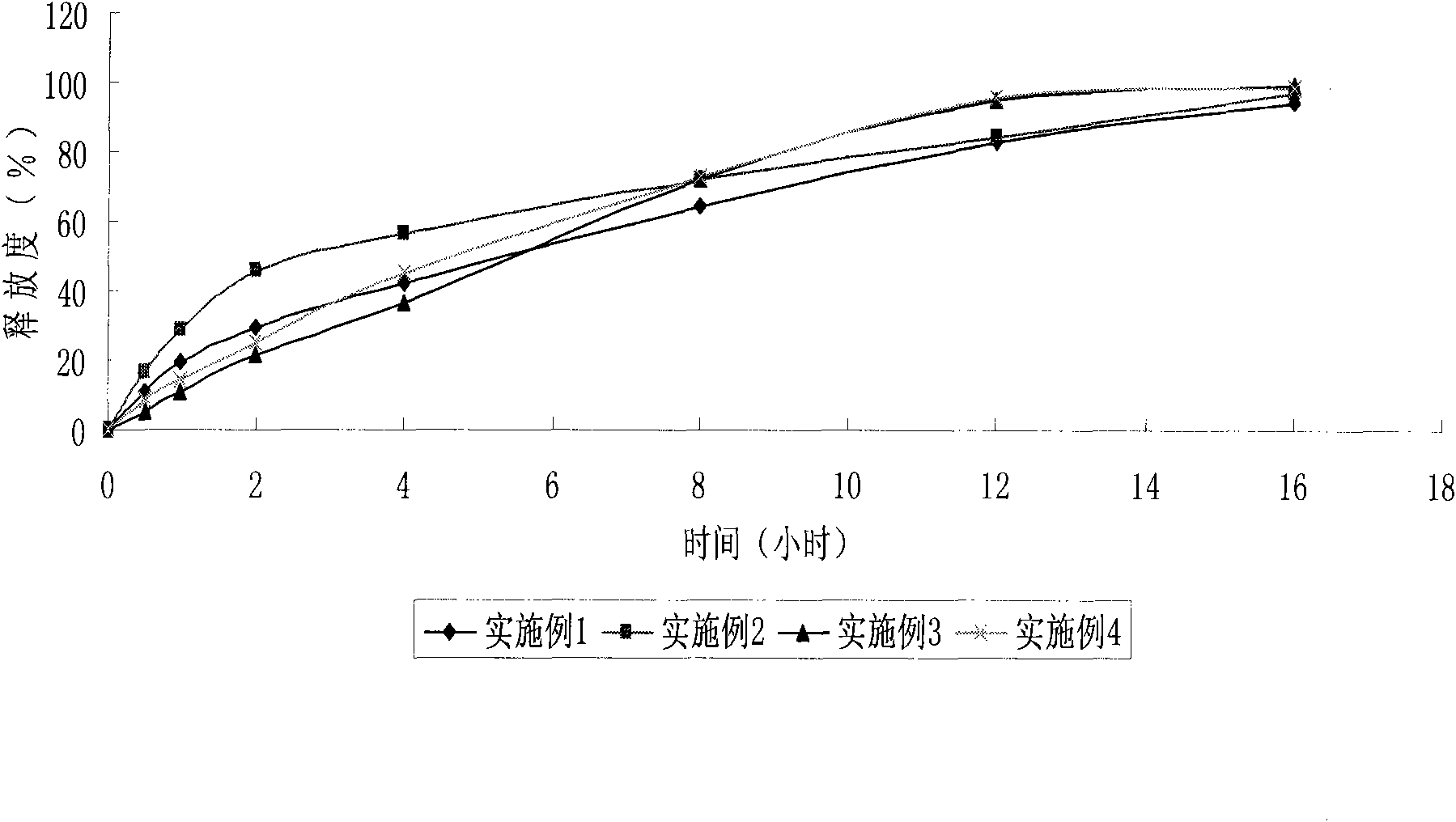
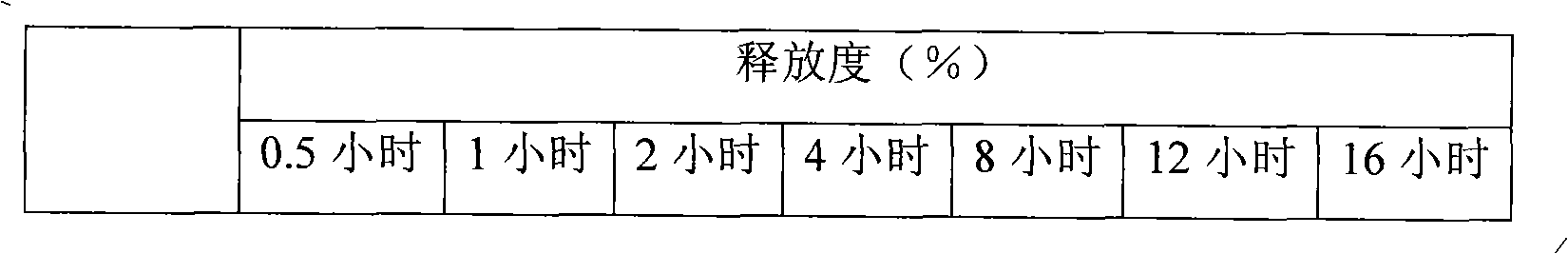
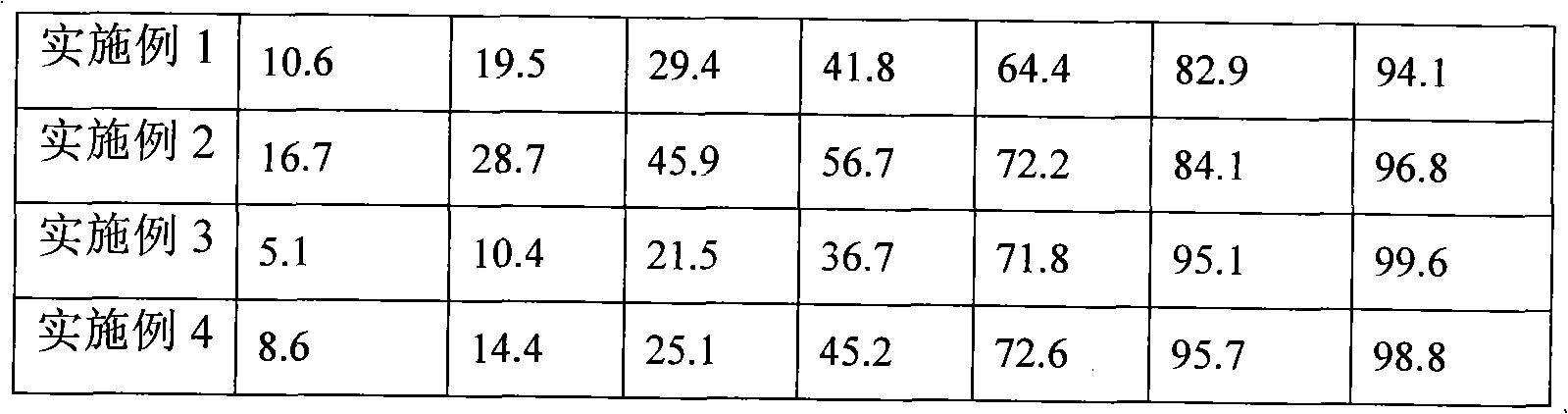
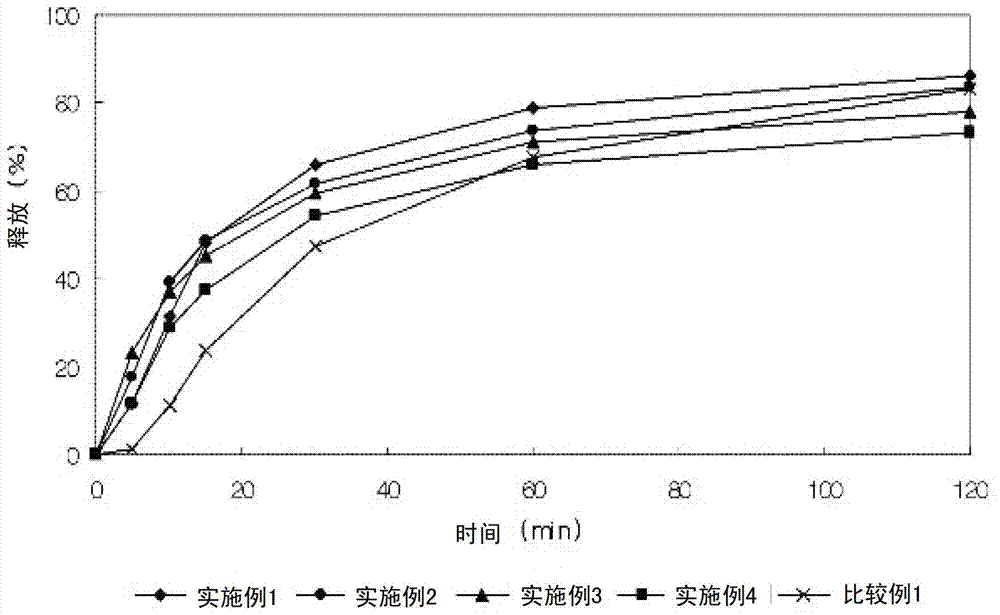
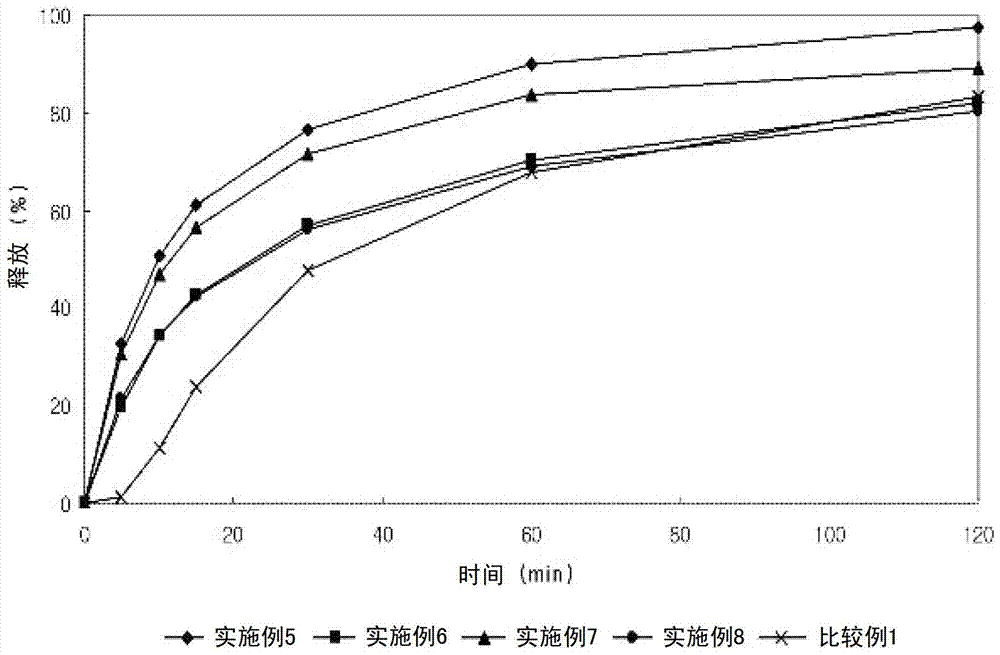
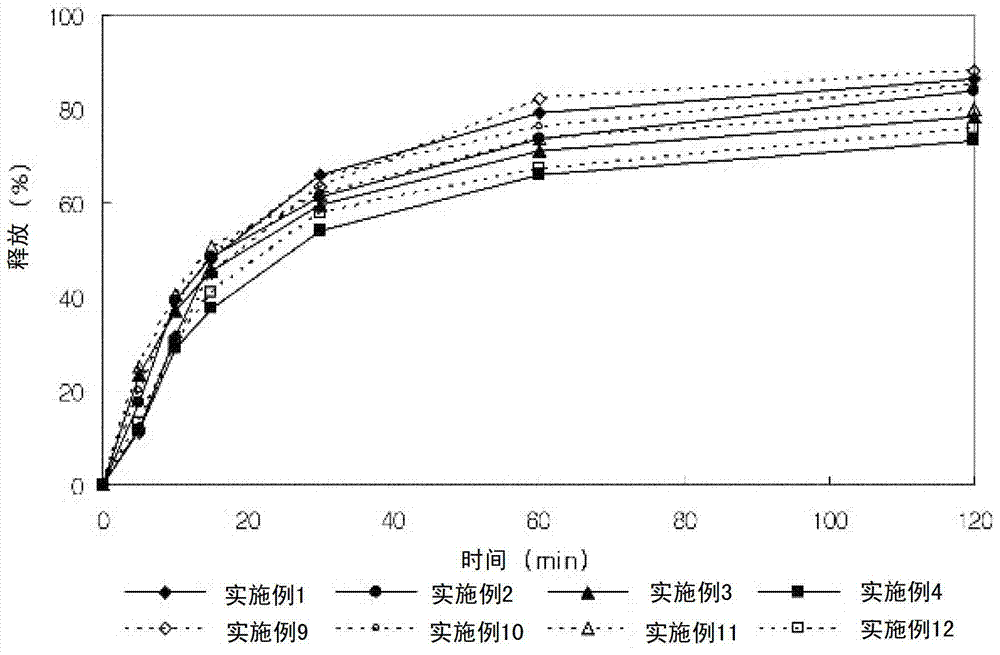
Lercanidipine hydrochloride sustained release preparation and preparation method thereof
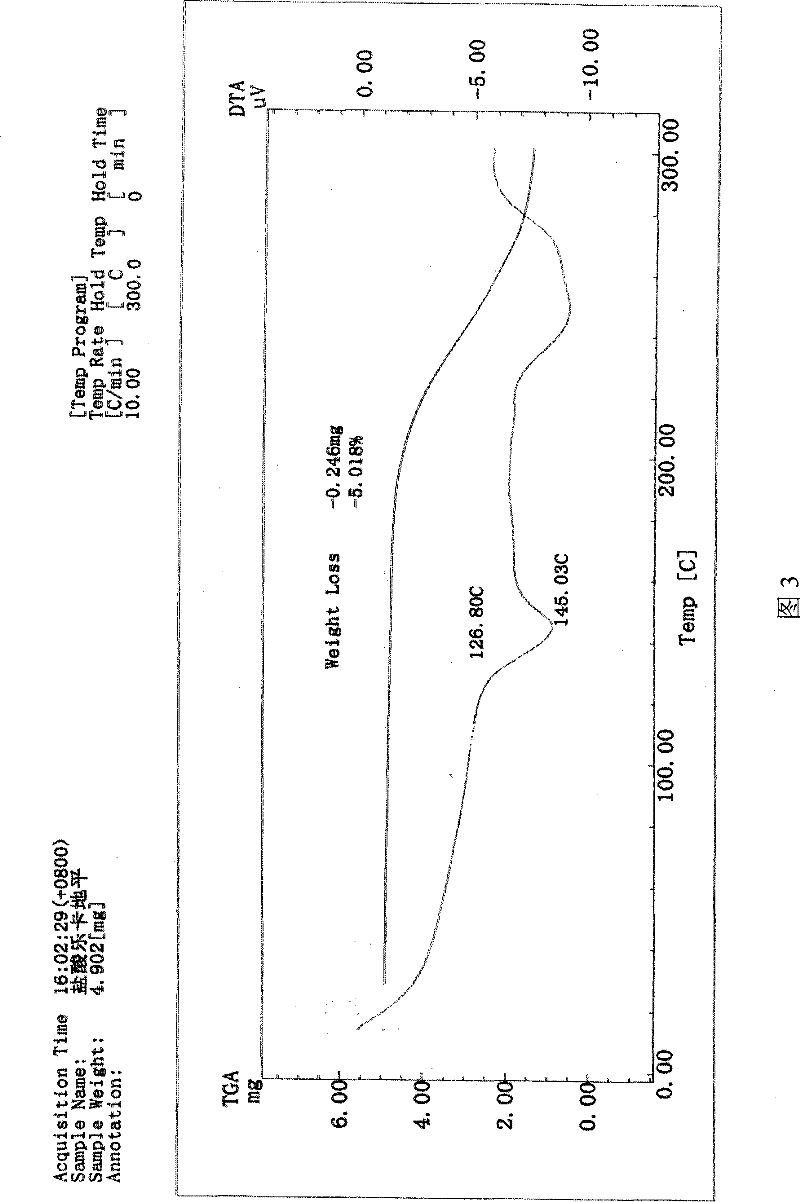
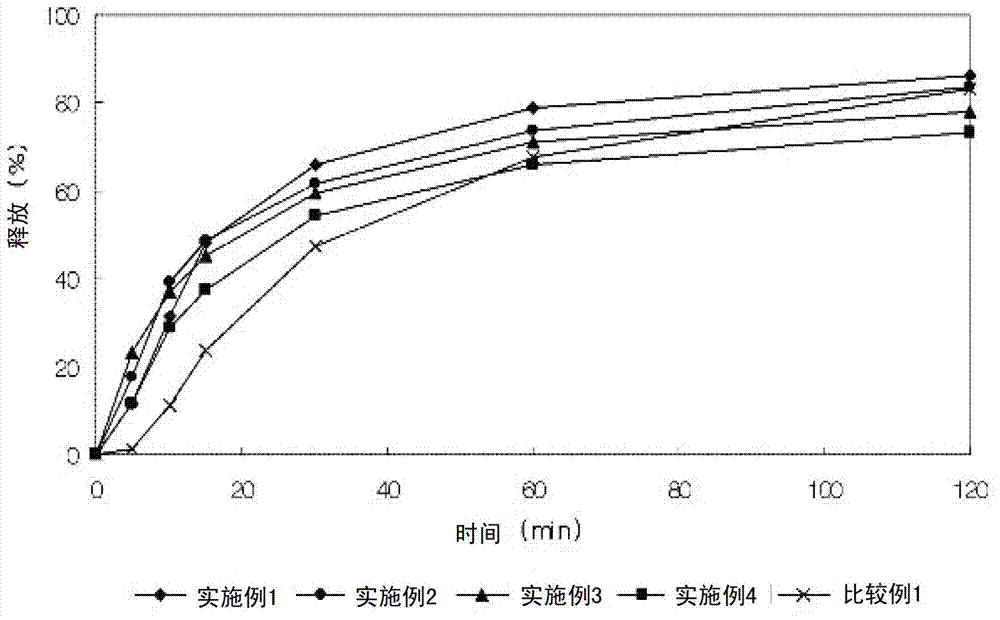
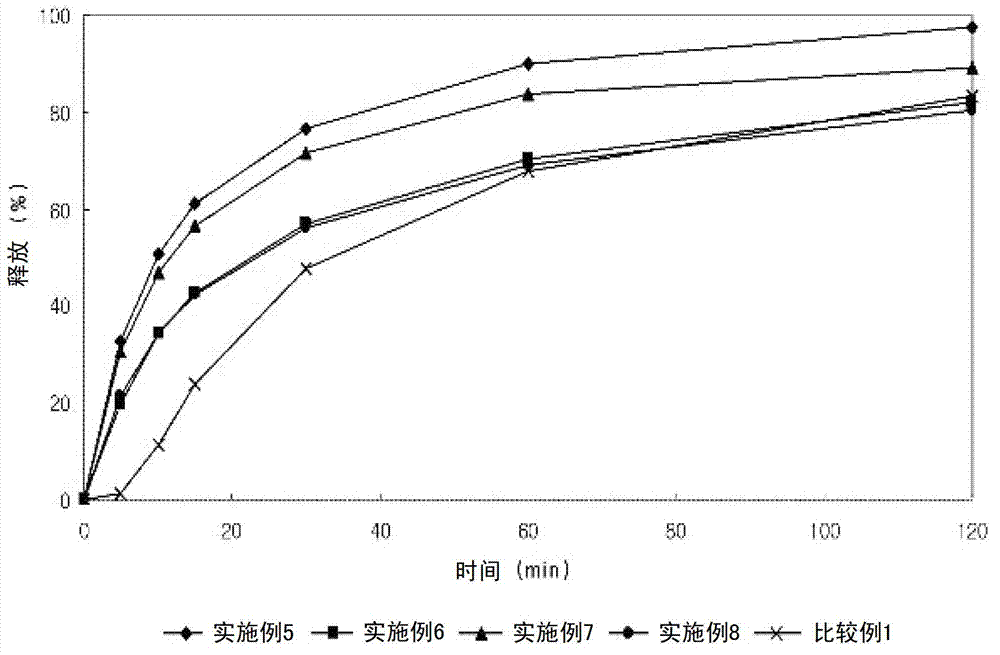
InactiveCN101658481APharmaceutical delivery mechanismCardiovascular disorderIn vivoPharmaceutical formulation
The invention discloses a sustained release medicinal preparation containing lercanidipine hydrochloride, which consists of an effective dose of lercanidipine hydrochloride and pharmaceutically acceptable pharmaceutic adjuvant. The lercanidipine hydrochloride sustained release medicinal preparation prepared can enable the medicament constantly and stably to release in vivo, ensures a steady plasmalevel, and radically improves the safety and effectiveness of the medicament.
Owner:COSCI MED TECH CO LTD
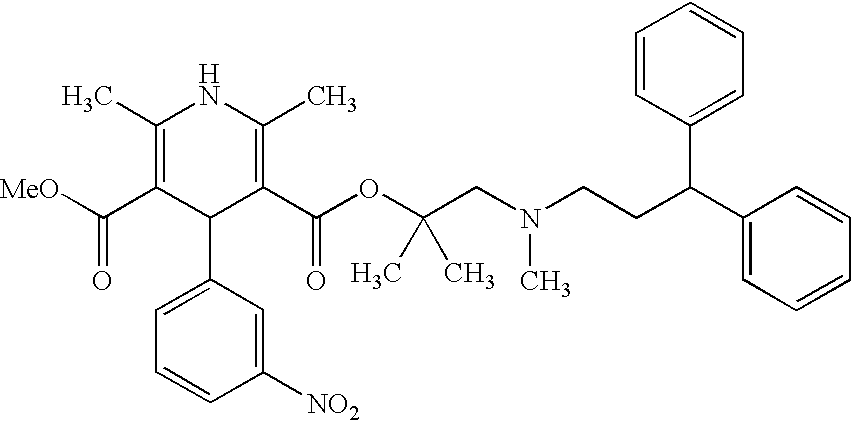
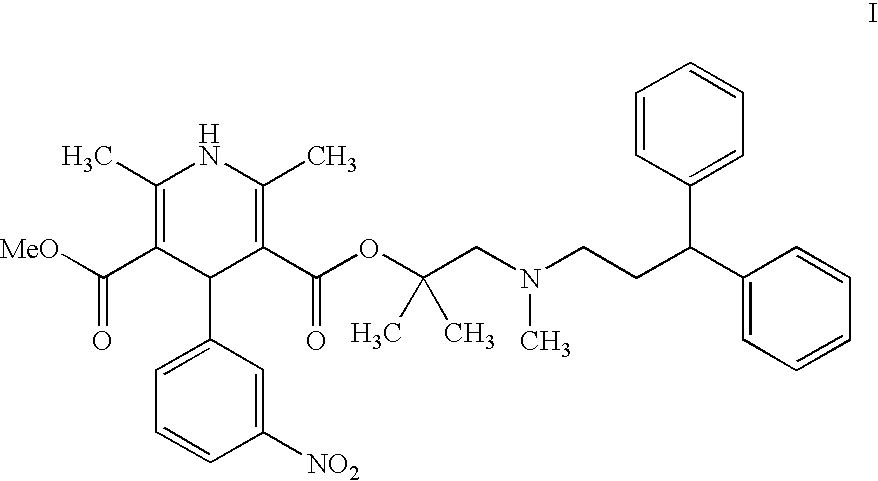
Novel process for the preparation of lercanidipine
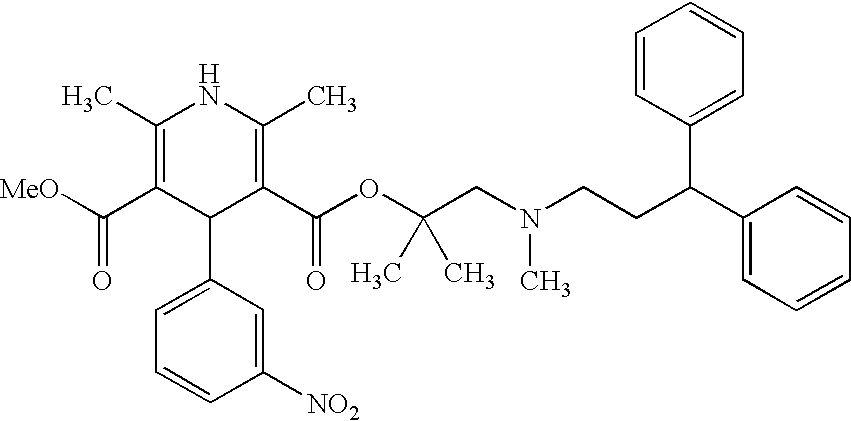
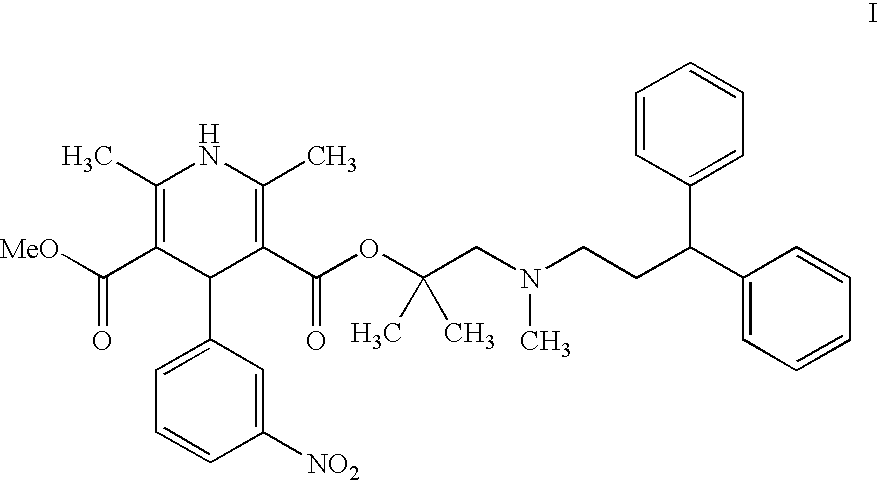
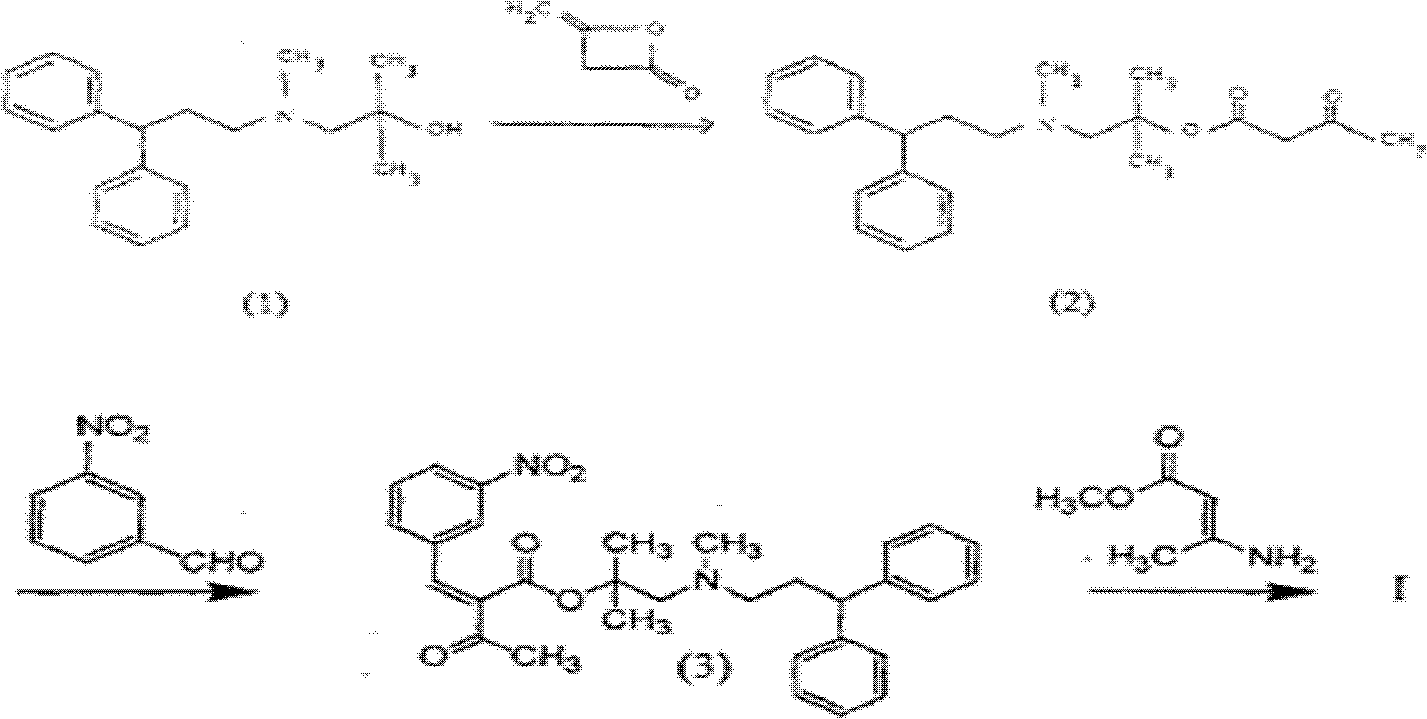
The invention provides a novel process for the preparation of lercanidipine or a pharmaceutical acceptable salt using novel intermediates. Thus, 2,N-dimethyl-N-(3,3-diphenylpropyl)-1-amino-2-propanol is reacted with trimethylsilyl chloride in presence of triethyl amine in methylene chloride to give 2,N-dimethyl-2-(trimethylsilyloxy)-N-(3,3-diphenylpropyl)-1-propanamine, which is then reacted with 2,6-dimethyl-5-methoxycarbonyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carbonyl chloride for 2 hours and crystallized to obtain lercanidipine hydrochloride.
Owner:HETERO DRUGS LTD
Composite preparation comprising lercanidipine hydrochloride and valsartan and preparation method thereof
ActiveCN103249415BLittle side effectsOvercoming problems caused by simple mixturesPill deliveryGranular deliveryValsartanActive component
The invention relates to a pharmaceutical composition containing lercanidipine hydrochloride and valsartan as active components and a preparation method thereof. The pharmaceutical composition comprising lercanidipine hydrochloride and valsartan of the present invention has excellent effects on preventing and treating cardiovascular diseases and their concurrent diseases, and reduces the side effects of each component. Furthermore, the composition of the present invention comprises lercanidipine hydrochloride and valsartan in isolated form to increase the dissolution rate of both components and reduce side effects.
Owner:LG CHEM LTD
Amorphous lercanidipine hydrochloride and preparation method thereof
ActiveCN102558032AEasy to joinImprove stabilityOrganic active ingredientsOrganic chemistryDihydropyridineAngina
The invention provides amorphous lercanidipine hydrochloride and a preparation method thereof. Lercanidipine hydrochloride is a third-generation dihydropyridine calcium antagonist and is mainly used for hypertension and angina in clinic. The invention provides amorphous lercanidipine hydrochloride with the purity being at least 98.5%, particularly at least 99.5% and more particularly at least 99.7%. The invention also provides a preparation method of high-purity amorphous lercanidipine hydrochloride.
Owner:CHINA RESOURCES SAIKE PHARMA
A kind of pharmaceutical composition for treating hypertension
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +1
Process for the preparation of lercanidipine
The invention provides a novel process for the preparation of lercanidipine or a pharmaceutical acceptable salt using novel intermediates. Thus, 2,N-dimethyl-N-(3,3-diphenylpropyl)-1-amino-2-propanol is reacted with trimethylsilyl chloride in presence of triethyl amine in methylene chloride to give 2,N-dimethyl-2-(trimethylsilyloxy)-N-(3,3-diphenylpropyl)-1-propanamine, which is then reacted with 2,6-dimethyl-5-methoxycarbonyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carbonyl chloride for 2 hours and crystallized to obtain lercanidipine hydrochloride.
Owner:HETERO DRUGS LTD
Polymorphic form of lercanidipine hydrochloride and process for the preparation thereof
ActiveUS8097729B2Improve efficiencyLess-waste is producedBiocideOrganic compounds purification/separation/stabilisationAlcoholIsopropyl acetate
Processes for the purification of lercanidipine hydrochloride are provided which uses a binary system of an alcohol-containing solvent such as methanol and an aliphatic ester-containing solvent such as isopropyl acetate. Processes for the preparation of substantially amorphous lercanidipine hydrochloride are also provided. Also provided is lercanidipine hydrochloride substantially in polymorph form V.
Owner:GLENMARK LIFE SCI LTD
Crystal form of lercanidipine hydrochloride and preparation method thereof and crystal form-containing medicinal composition
ActiveCN102020602BHigh purityImprove stabilityOrganic active ingredientsOrganic chemistryX-rayX ray diffractogram
The invention provides a novel crystal form of lercanidipine hydrochloride, which has peaks at 5.38 DEG, 10.74 DEG, 11.54 DEG, 11.88 DEG, 15.14 DEG, 17.26 DEG, 17.92 DEG, 19.82 DEG, 20.22 DEG, 20.70 DEG, 21.44 DEG, 21.98 DEG and 22.56 DEG expressed by a 2theta angle in an X ray diffraction pattern. The novel crystal form has high purity (over 99.5 percent) and high stability, has low rigidity and is easy to crush after being dried, and facilitates preparing and using a medicinal composition. The invention also provides a preparation method for the crystal form and a crystal form-containing medicinal composition. Compared with the prior art, the preparation method has the advantages of simple process, mild preparation conditions and high yield.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Lercanidipine hydrochloride crystal and preparation method thereof
The invention is a lercandipine hydrochloride crystal (form (HX) crystal) and a preparation method. The crystal has no crystalline water and virgule or crystalline solvent, and is a novel high-purity stable crystalline form, which is more suitable for the large-scale industrial production. The invention has advantages of simple and easy operation, and facilities to prepare the drug combination and provides the clinical use. The crystal uses the Cu-K alpha radiation. The X-ray powder diffraction spectra show expressed in the 2-theta angle has the following characteristics: an angle (2 theta), D(A), a peak relative intensity (%): 7.4, 11.9, 47; 9.6, 9.2, 64; 12.6, 7.0, 32; 13.2, 6.7, 36; 15.7, 5.6, 38; 15.9, 5.6, 63; 17.0, 5.2, 64; 21.4, 4.1, 100; 23.6, 3.8, 87; 5, 3.6, 58.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD
Complex formulation comprising lercanidipine hydrochloride and valsartan and method for the preparation thereof
ActiveCN103249415ALittle side effectsOvercoming problems caused by simple mixturesPill deliveryGranular deliveryValsartanActive component
The present invention relates to a pharmaceutical composition comprising lercanidipine hydrochloride and valsartan as active components and a method for the preparation thereof. The pharmaceutical composition comprising lercanidipine hydrochloride and valsartan according to the present invention has a superior effect on the prevention and treatment of cardiovascular diseases and their complex diseases, and reduces the adverse effects of each component. In addition, the present composition comprises lercanidipine hydrochloride and valsartan in a separated form so as to increase the dissolution rates of both components and reduce the adverse effects.
Owner:LG CHEM LTD
Amorphous lercanidipine hydrochloride and uses thereof
ActiveUS7820701B2Good water solubilityRapid onsetBiocideOrganic compounds purification/separation/stabilisationPharmacologyLercanidipine Hydrochloride
The invention provides a substantially pure amorphous lercanidipine hydrochloride having a purity of at least 95% pure, preferably at least about 97% pure, more preferably at least about 99% pure, and still more preferably at least about 99.5% pure. The invention further relates to methods of preparing substantially pure amorphous lercanidipine, as well as methods of providing rapid relief from hypertension by administering the substantially pure amorphous lercanidipine hydrochloride of the present invention to a patient in need of such treatment.
Owner:RECORDATI IRELAND LTD
Amorphous lercanidipine hydrochloride and preparation method thereof
Lercanidipine hydrochloride is the third generation of dihydrophridine calcium antagonist and mainly used for treating hypertension and angina clinically. The amorphous lercanidipine hydrochloride is easy to smash, and the purity of the amorphous lercanidipine hydrochloride is at least 98.5%, higher purity is at least 99.5%, and the highest purity is at least 99.7%. The invention further provides a preparation method for the high purity amorphous lercanidipine hydrochloride.
Owner:CHINA RESOURCES SAIKE PHARMA
Synthesis process for high-purity lercanidipine hydrochloride
Owner:CHINA RESOURCES SAIKE PHARMA
Novel crystalline polymorphic forms of lercanidipine hydrochloride and process for their preparation
Owner:RECORDATI IRELAND LTD
Polymorphic Form of Lercanidipine Hydrochloride and Process for the Preparation Thereof
ActiveUS20090221833A1Improve efficiencyLess-waste is producedOrganic active ingredientsOrganic compounds purification/separation/stabilisationPurification methodsAlcohol
Processes for the purification of lercanidipine hydrochloride are provided which uses a binary system of an alcohol-containing solvent such as methanol and an aliphatic ester-containing solvent such as isopropyl acetate. Processes for the preparation of substantially amorphous lercanidipine hydrochloride are also provided. Also provided is lercanidipine hydrochloride substantially in polymorph form V.
Owner:GLENMARK LIFE SCI LTD
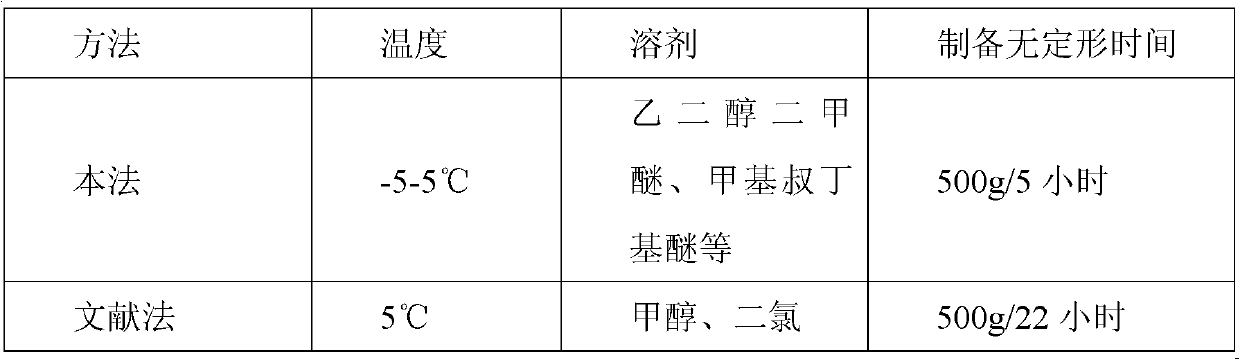
Refinement method of lercanidipine hydrochloride
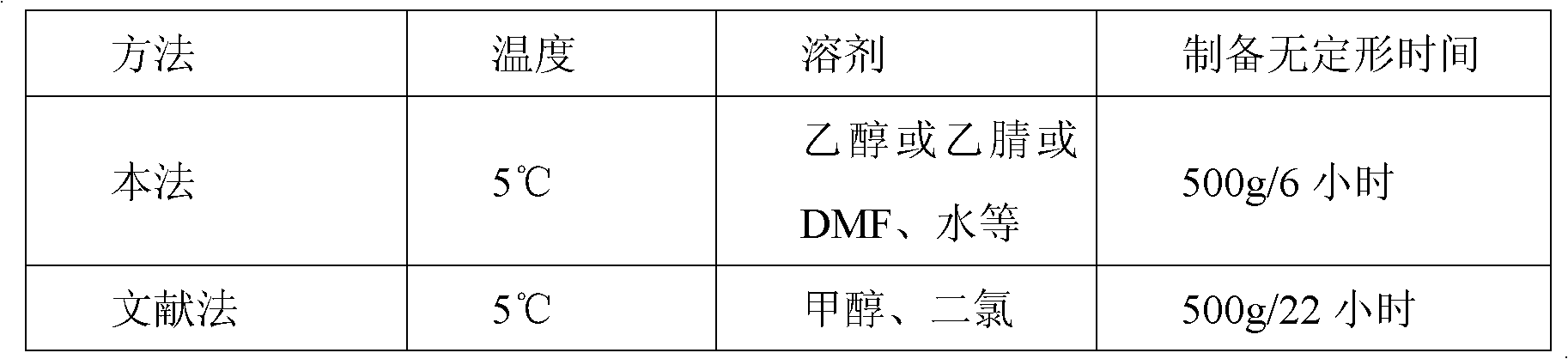
The invention discloses a refinement method of lercanidipine hydrochloride, which comprises the following steps: dissolving a lercanidipine hydrochloride crude product in mixed organic solvent of C1-3 alkyl alcohol and C1-3 alkyl acetate, wherein the volume mass ratio of the mixed organic solvent is 2-6 times, preferably 3-4 times; heating to 0-40 DEG C, and stirring, wherein the preferable temperature is 30-35 DEG C; soaking and washing for 2 hours, and cooling to 0-25 DEG C, preferably 18-25 DEG C; and continuing stirring the obtained solution for 6 hours, filtering the precipitated solid, washing the filter cake with absolute ethanol to obtain a yellow solid, and carrying out forced air drying to obtain the high-purity lercanidipine hydrochloride. The method further refines the lercanidipine hydrochloride crude product, is simple and convenient for operation, has the advantages of high purification rate, high speed, high stability in the whole operation, short refinement period, high yield and cost saving, and finally obtains the high-purity lercanidipine hydrochloride.
Owner:CHINA RESOURCES SAIKE PHARMA
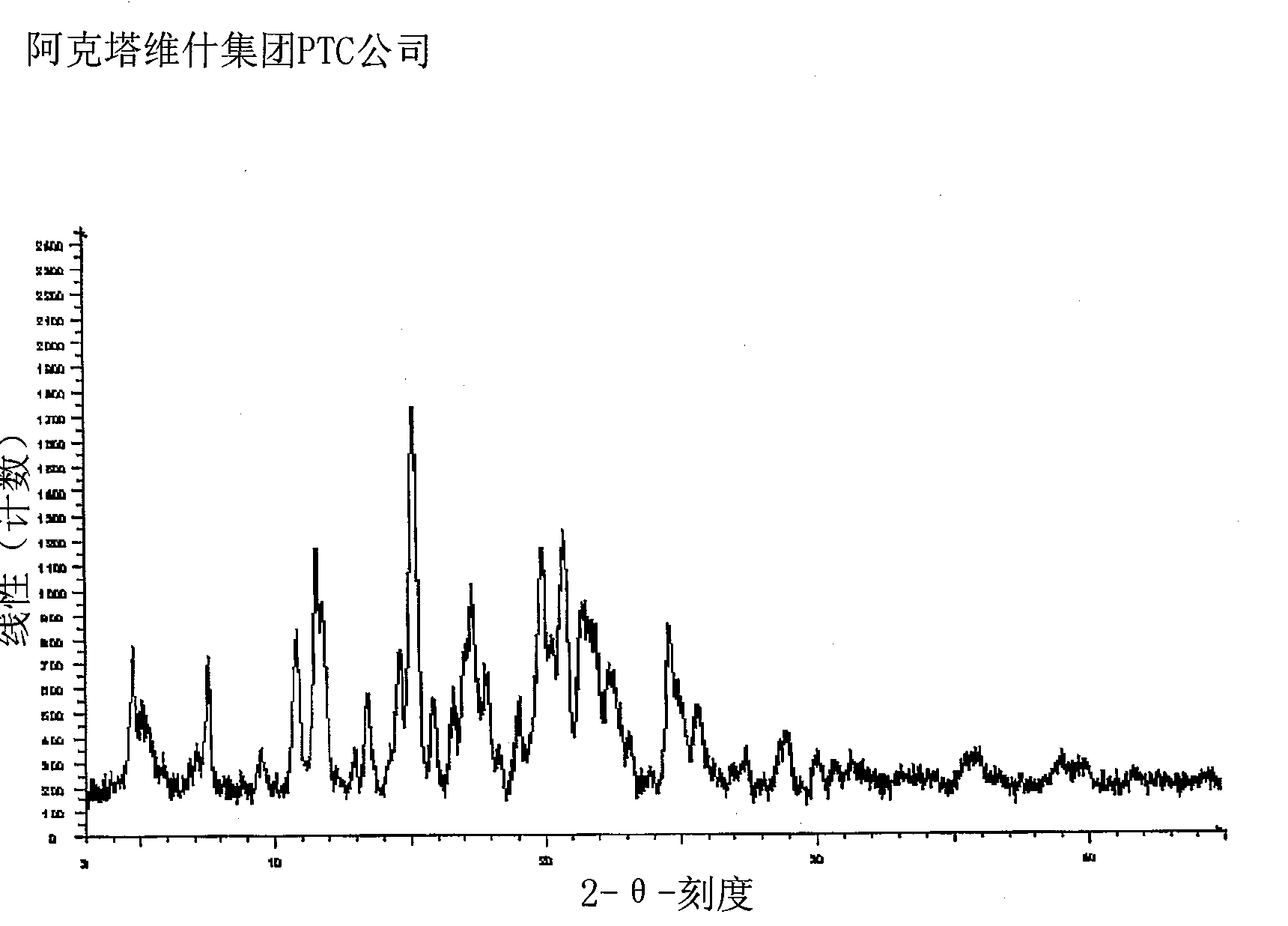
Lercanidipine Hydrochloride Polymorphs and an Improved Process for Preparation of 1,1,N-Trimethyl-N-(3,3-Diphenylpropyl)-2-Aminoethyl Acetoacetate
Disclosed herein is an improved, commercially viable and industrially advantageous process for the preparation of substantially pure Lercanidipine intermediate, 1,1,N-trimethyl-N-(3,3-diphenylpropyl)-2-aminoethyl acetoacetate. The intermediate is useful for preparing Lercanidipine, or a pharmaceutically acceptable salt thereof, in high yield and purity. The present invention further provides a novel crystalline form of Lercanidipine hydrochloride and a process for its preparation. The present invention also provides a process for the preparation of amorphous form of Lercanidipine hydrochloride.
Owner:ACTAVIS GRP PTC EHF
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com