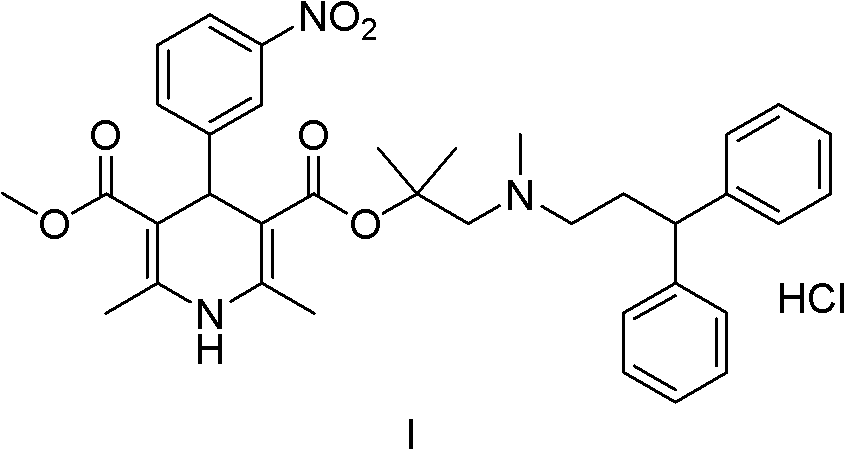
Synthesis process for high-purity lercanidipine hydrochloride
A technology of lercanidipine hydrochloride and synthesis process, applied in the field of pharmacy, can solve the problems of long cycle, complicated operation, difficult preparation and the like, and achieve the effects of mild conditions, simple operation and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
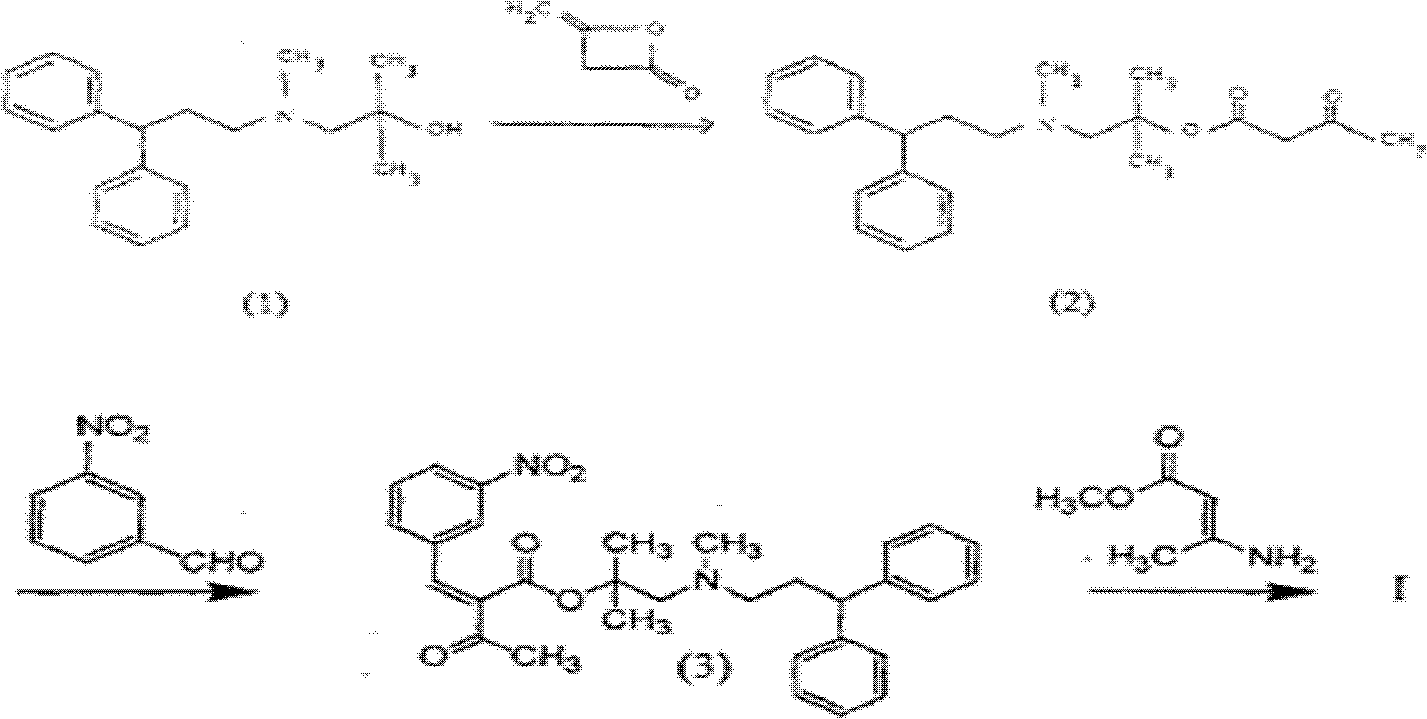
[0049] Embodiment 1, the preparation of propionitrile acetoacetate
[0050]Add 14.2g of 3-hydroxypropionitrile and 0.2g of ethylenediamine into the dry reaction flask, add 18g of diketene dropwise at room temperature under full stirring, and control the temperature not higher than 75°C. After stirring and reacting for 2 hours, put the reaction solution in Evaporate under reduced pressure at 70°C until there is no distilled matter to obtain a light yellow oil, add 60ml of dichloromethane, wash twice with 50ml of water, once with 50ml of saturated brine, dry over anhydrous sodium sulfate, filter with suction, and evaporate to dryness under reduced pressure at 45°C. 30 g of light yellow oil was obtained, which was directly used for the next reaction.
Embodiment 2
[0051] Embodiment 2, the synthesis of lercanidipine mother nucleic acid
[0052] Add 15.6g of propionitrile acetoacetate, 17.5g of 3-nitrobenzaldehyde, 50ml of isopropanol, a mixed solution of 0.5g of piperidine and 0.3g of acetic acid into the dry reaction flask, and add 12.593- Aminobutyrylic acid methyl ester, stir and react at room temperature to 50°C for 12 hours, cool down to room temperature (<25°C), add 20ml of newly prepared 5.8gKOH in isopropanol solution in a water bath, stir for 2 hours, and depressurize at 45°C Evaporate the solvent, add 50ml of water and stir at 50°C for 1 hour, collect the water phase, repeat 3 times, combine the water phase and adjust the pH to 3-4 with 4M hydrochloric acid while stirring in a water bath, a large amount of solids are precipitated, suction filtered, and the filter cake is washed with water After three times until the pH was stable, 23.6 g of a white to light yellow solid was obtained by crystallization with methanol, with a yiel...
Embodiment 3
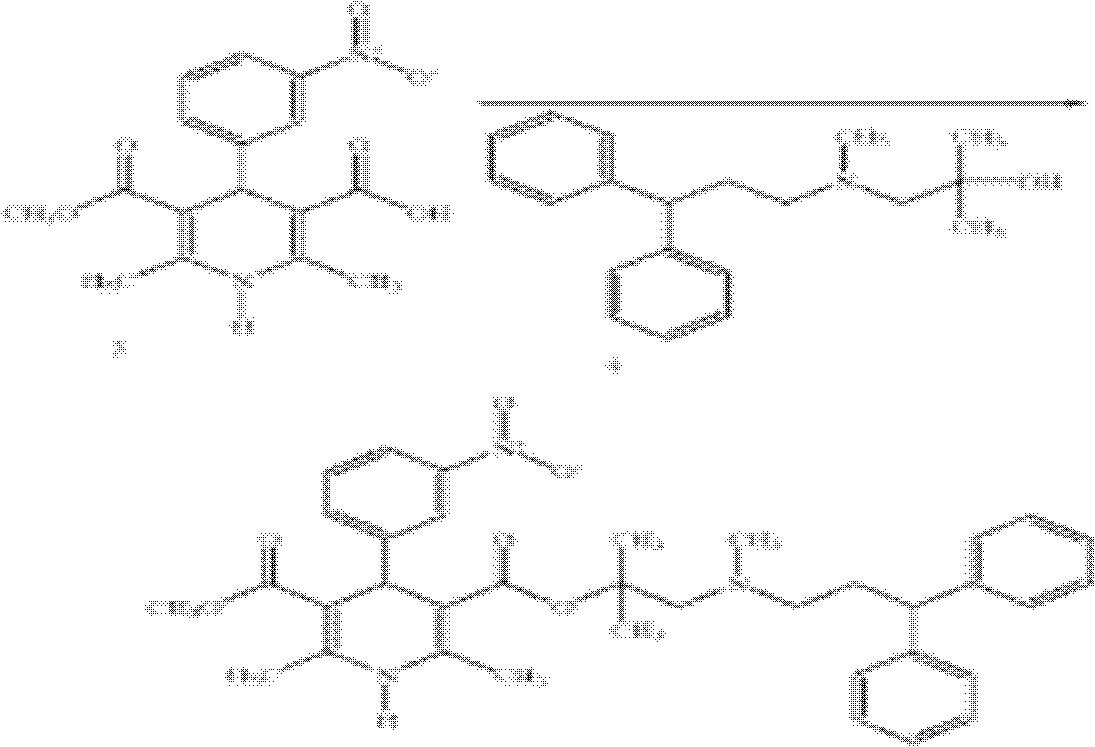
[0053] The synthesis of embodiment 3 lercanidipine hydrochloride
[0054] In dry reaction bottle, add 16.7g (0.05mol) mother nucleus, 90mlDCM, 3mlDMF, in N 2 Under protection, 5.5ml (0.075mol) of thionyl chloride was added dropwise at 0°C-10°C, stirred at this temperature for 2-3 hours, 17.1g (0.06mol) of the side chain was added dropwise, stirred for 5 hours, TLC showed After the reaction is complete, add 100ml of water, separate the liquids, wash the organic phase twice with 100ml of water, once with saturated brine, dry over anhydrous sodium sulfate, filter with suction, evaporate to dryness under reduced pressure to obtain an oily product, add 50ml of DME and stir overnight to obtain a light yellow solid. After filtration and vacuum drying at 60°C, 19.6 g of a light yellow solid was obtained by crystallization from ethanol, with a yield of 63.8% and a purity of 99.82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com