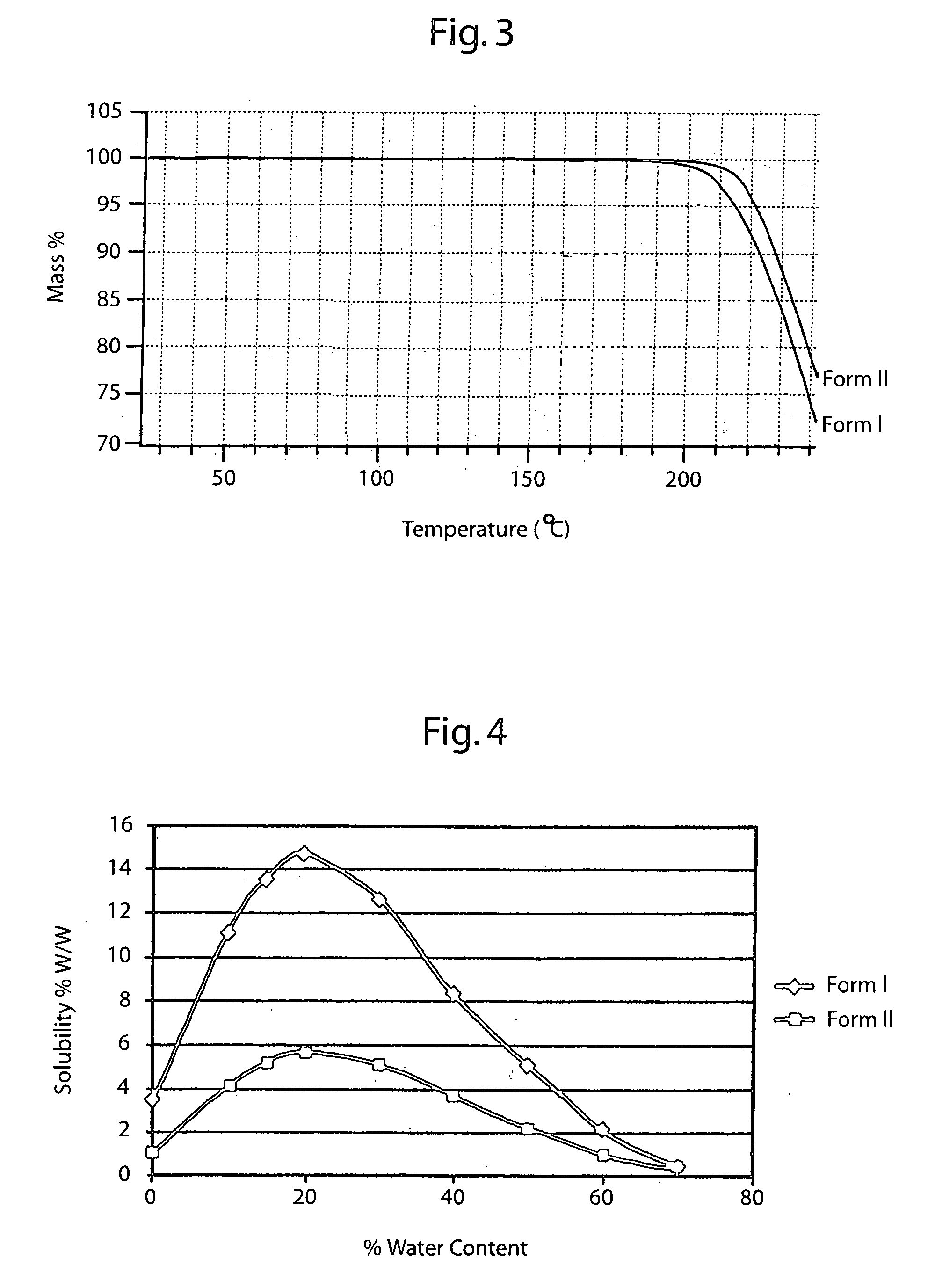
Novel crude and crystalline forms of lercanidipine hydrochloride
a technology of lercanidipine and lercanidipine, which is applied in the field of new crude forms and crystalline forms of lercanidipine hydrochloride, can solve the problems of difficult industrial scale complex purification and isolation steps of lercanidipine from reaction mixtures, and low yield of desired products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Initial Preparation
[0149] Thionyl chloride (36 g) diluted in ethyl acetate (25 g) was slowly added to a solution of 2,6-dimethyl-5-methoxycarbonyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylic acid (90 g) prepared, e.g., as disclosed in German patent DE 2847 237, in dimethylformamide (115 g) and ethyl acetate (396 g), keeping temperature between −1 and +1° C. A solution of 2, N-dimethyl-N-(3,3-diphenylpropyl)-1-amino-2-propanol (84 g) in ethyl acetate (72 g) was slowly added to the mixture thus obtained. The whole was kept under stirring at the same temperature for 3 hours. The mixture was then heated to 20-25° C. and kept under stirring for 12 hours. Water (340 ml) was then added, the whole was stirred for 30 min and after settling the aqueous phase was discarded. The organic phase was washed again with water (340 ml).
example 2
Crude Lercanidipine Hydrochloride Form (A)
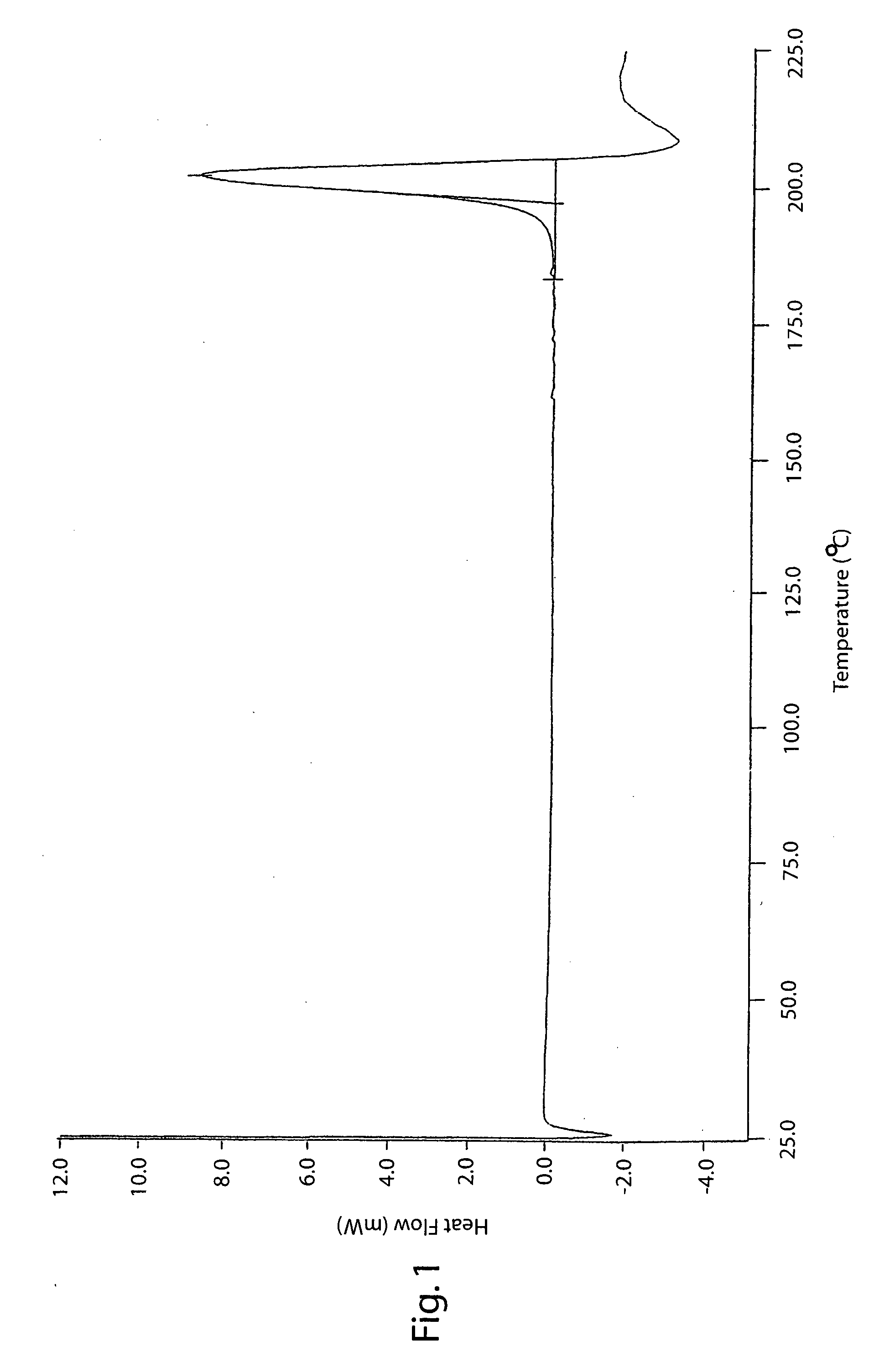
[0150] The organic phase obtained from Example 1 was then subjected to azeotropic distillation under vacuum at about 250 mmHg, without going above a temperature of 60° C. After removing about 50 ml of water, the solution was concentrated to about ⅓ of the initial volume in the same conditions of temperature and pressure and then brought to its initial volume with fresh ethyl acetate until the K. F. value (Karl Fisher value) was about 0.10-0.15%. The final suspension was cooled to 0-5° C. The solid was filtered, suspended in ethyl acetate (350 g) and stirred at 60-65° C. for 1 hour. The whole was cooled to 5-10° C. and then filtered. The solid was dried in an oven at 70° C. 133 g of dry raw lercanidipine hydrochloride Form (A) was obtained (75% yield), DSC peak 150-152° C.
example 3
Crude Lercanidipine Hydrochloride Form (B)
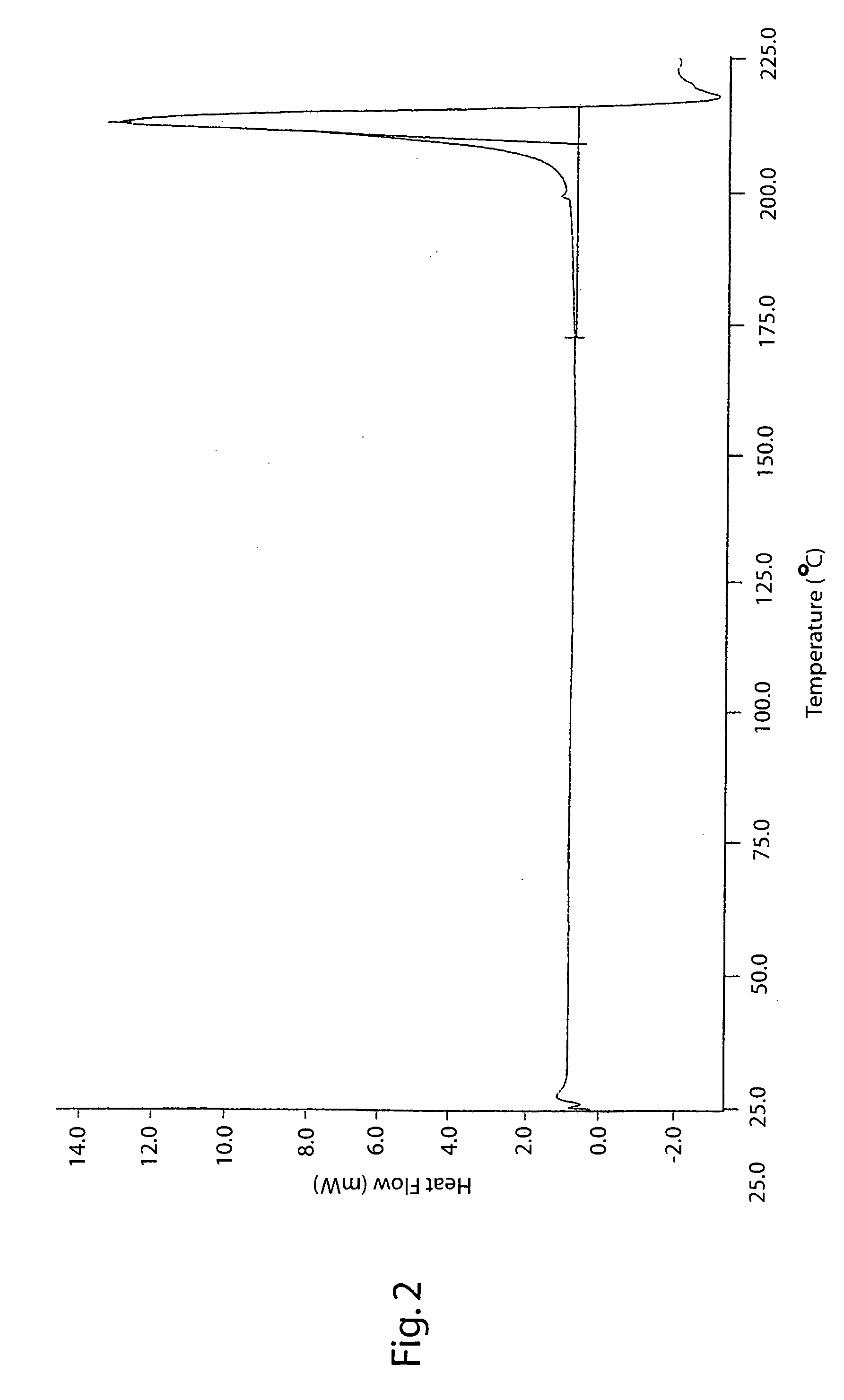
[0151] The organic phase obtained at the end of Example 1 was heated under reflux (70-75° C.) and the water contained in the solution was removed with a Dean Stark apparatus (Spaziani Rolando, Nettuno, Rome, Italy) until a K.F. value of about 2% was obtained. The whole was then distilled at atmospheric pressure to reach ¾ of initial volume. The solution was brought to its initial volume by adding fresh ethyl acetate. The K.F. value at the end of this operation was 0.9-1.1%. The final solution was cooled to 0-5° C. A solid slowly precipitates which was filtered. The solid thus obtained was suspended in ethyl acetate (350 g) and stirred at 60-65° C. for 1 hour. The whole was cooled to 5-10° C., then filtered and dried in an oven at 70° C., thus obtaining 133 g of crude lercanidipine hydrochloride Form (B), DSC peak 131-135° C.; 75% yield.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com