Compound preparation of lercanidipine and atorvastatin
A technology of atorvastatin calcium and compound preparations, applied in the field of medicine, to achieve the effects of reducing adverse reactions, good clinical application prospects, and convenient medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
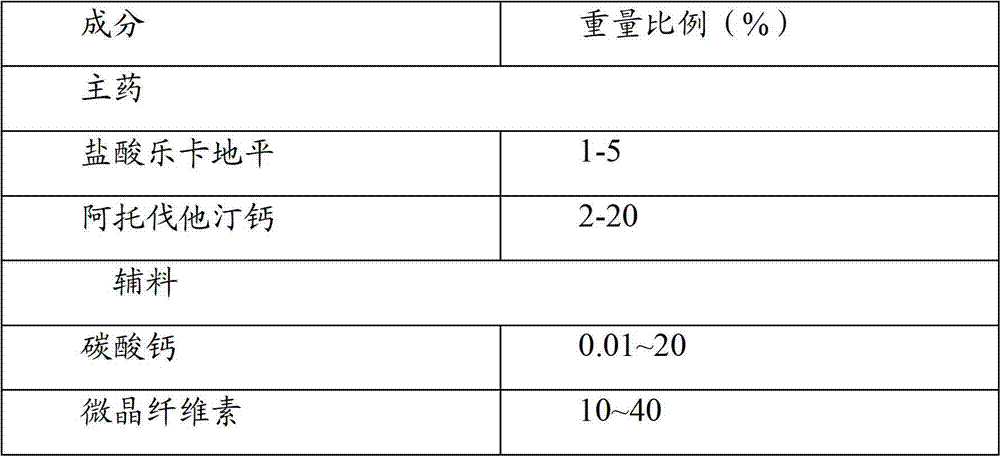
[0029] Embodiment 1: the prescription composition of compound preparation of the present invention
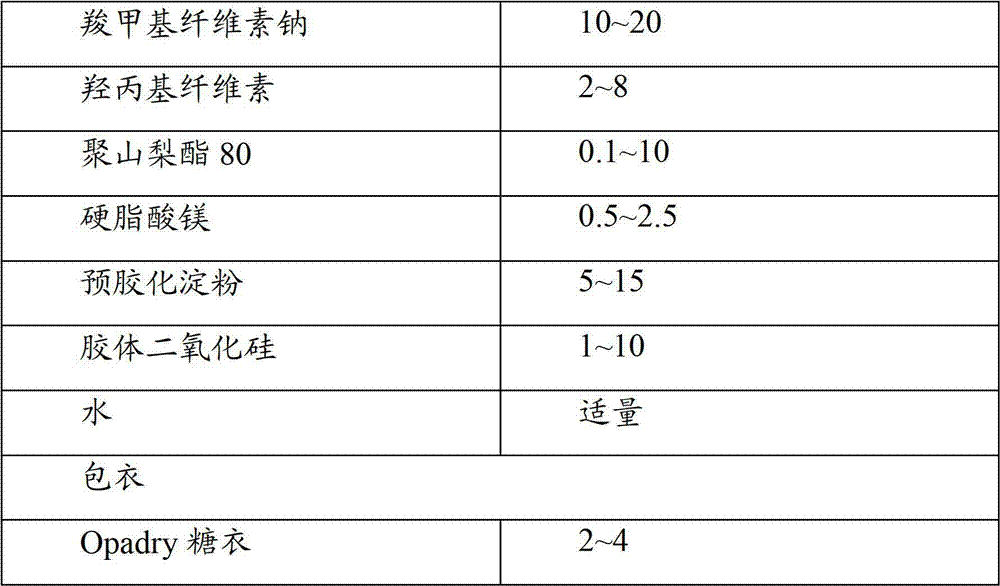
[0030] The compound preparation contains the main drugs lercanidipine hydrochloride and atorvastatin calcium, and the excipients are calcium carbonate, sodium carboxymethyl cellulose, microcrystalline cellulose, pregelatinized starch, polysorbate 80, hydroxypropyl cellulose, purified Water, Colloidal Silicon Dioxide, Magnesium Stearate, Opadry Icing.
[0031] Table 1 prescription composition
[0032]
[0033]
Embodiment 2
[0034] Embodiment 2: the preparation of compound tablet of the present invention
[0035] (1) Weigh lercanidipine hydrochloride raw materials and atorvastatin calcium raw materials and sieve them for later use.
[0036](2) Weigh calcium carbonate, microcrystalline cellulose, sodium carboxymethyl cellulose, hydroxypropyl cellulose, polysorbate 80, magnesium stearate, pregelatinized starch, colloidal silicon dioxide, and stir with raw materials Mix evenly; calcium carbonate 0.01-20% in auxiliary materials, microcrystalline cellulose 10-40%, sodium carboxymethyl cellulose 10-20%, hydroxypropyl cellulose 2-8%, polysorbate 800.1-10%, Magnesium stearate 0.5-2.5%, pregelatinized starch 5-15%, colloidal silicon dioxide 1-10%.
[0037] (3) Vacuum compression, milling, sieving and granulation; tableting, control tablet core hardness to 2-10kg.
[0038] (4) Film coating: Take Opadry (Y-1-7000), add it to ethanol under stirring condition, stir until dispersed, then add purified water an...
Embodiment 3
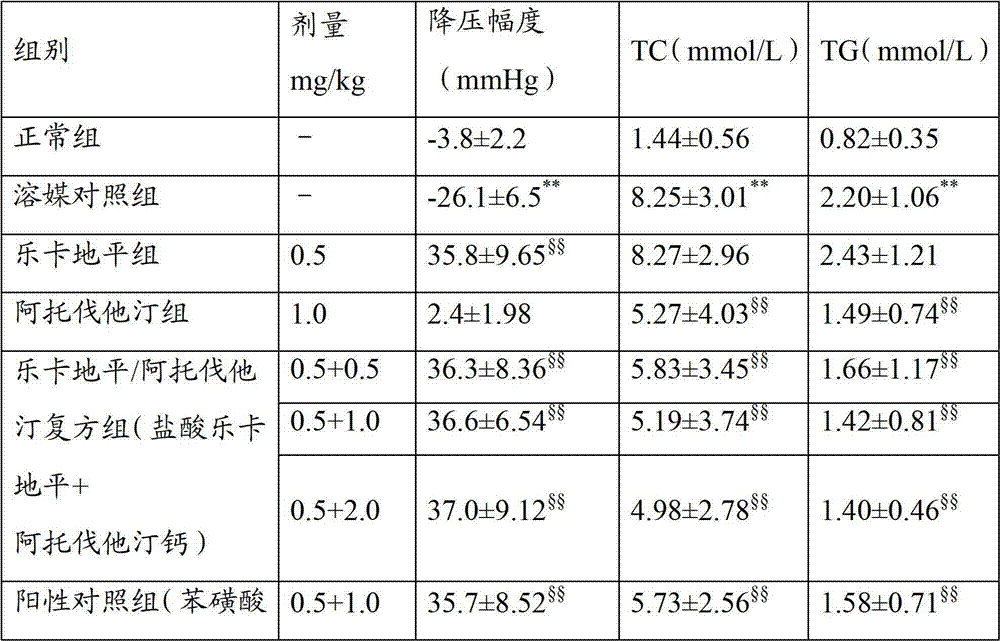
[0040] Example 3: Quality Research Methods
[0041] Including properties, identification, inspection (related substances, loss on drying, chloride, heavy metal, dissolution rate, content uniformity) and content determination, etc. Wherein the assay method of lercanidipine hydrochloride is as follows:
[0042] Content determination: HPLC method, the stationary phase and mobile phase used are as follows.
[0043] The HPLC assay condition of lercanidipine hydrochloride in the compound preparation of table 2
[0044] mobile phase
[0045] Preparation method of buffer A: dissolve 1.36g of potassium dihydrogen phosphate in 1L of water, add 1g of octane sulphonic acid sodium salt to it after dissolving, and use 10% phosphoric acid (v / v) for ultrasonic treatment Adjust the pH to 2.5 ± 0.05.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com