Novel crystalline polymorphic forms of lercanidipine hydrochloride and process for their preparation
A technology for lercanidipine hydrochloride and lerca hydrochloride, which is applied in the directions of solution crystallization, medical preparations containing active ingredients, and drug combinations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0158] Embodiment 2 thick lercanidipine hydrochloride shape (A)
Embodiment 1
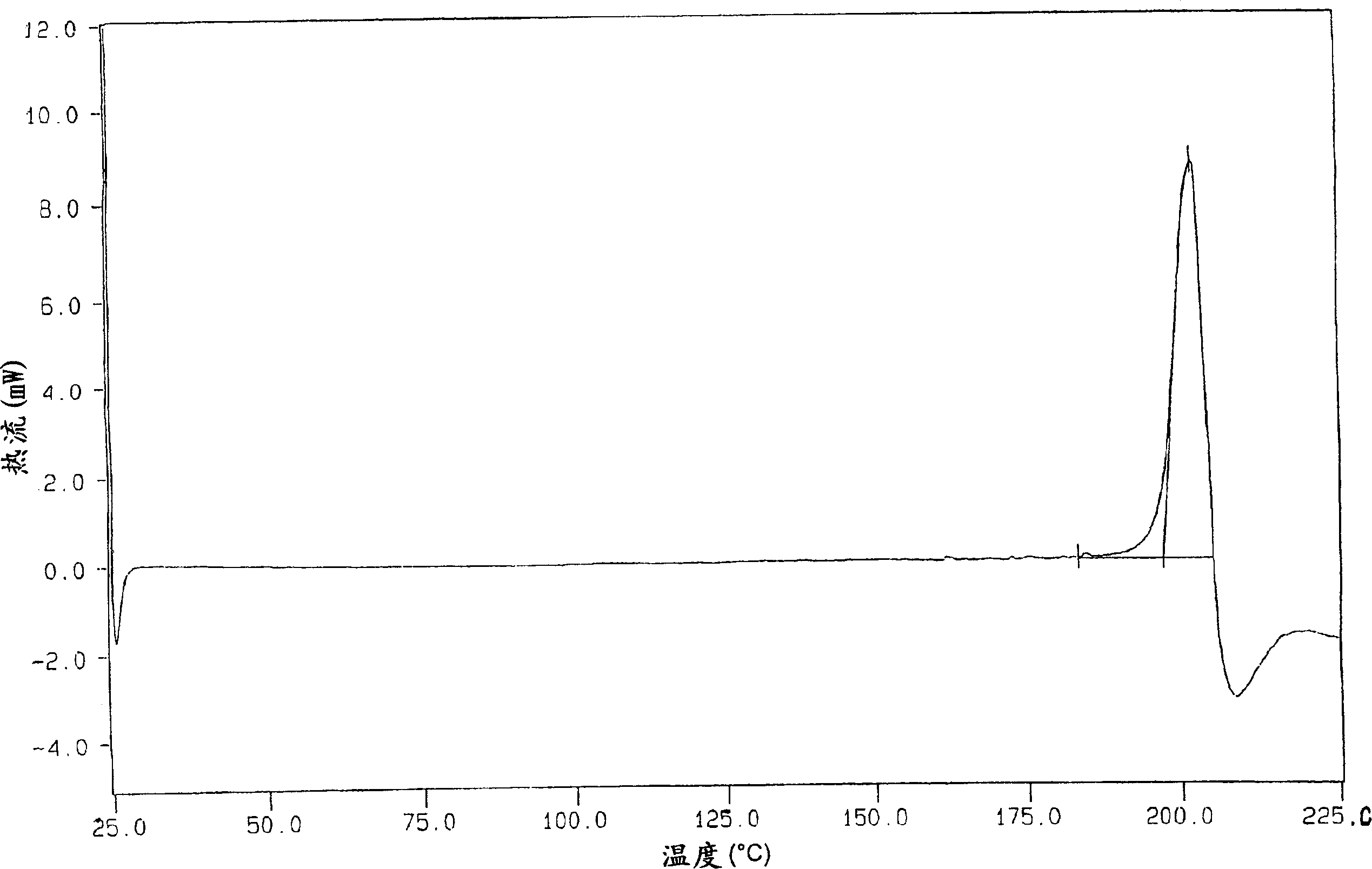
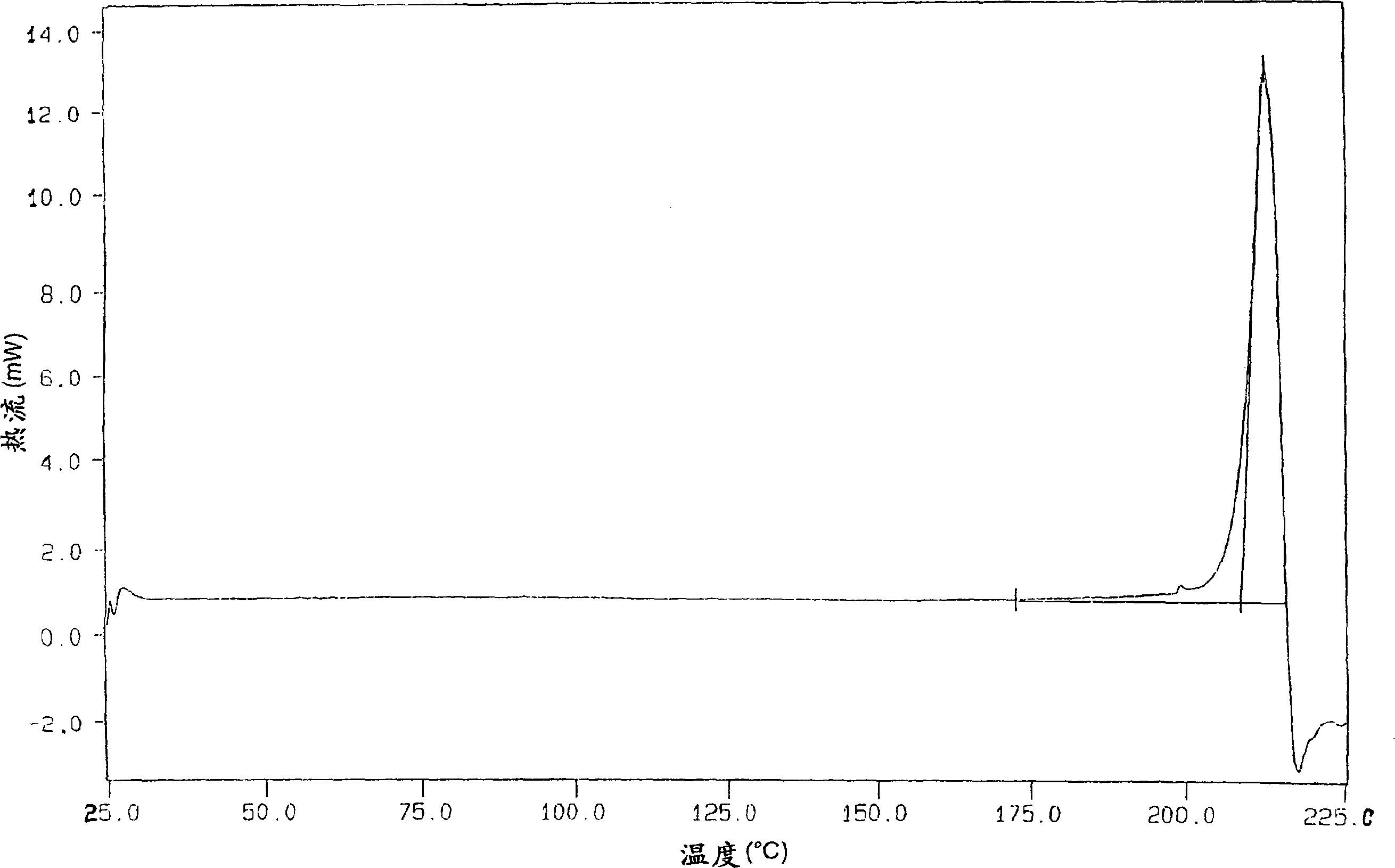
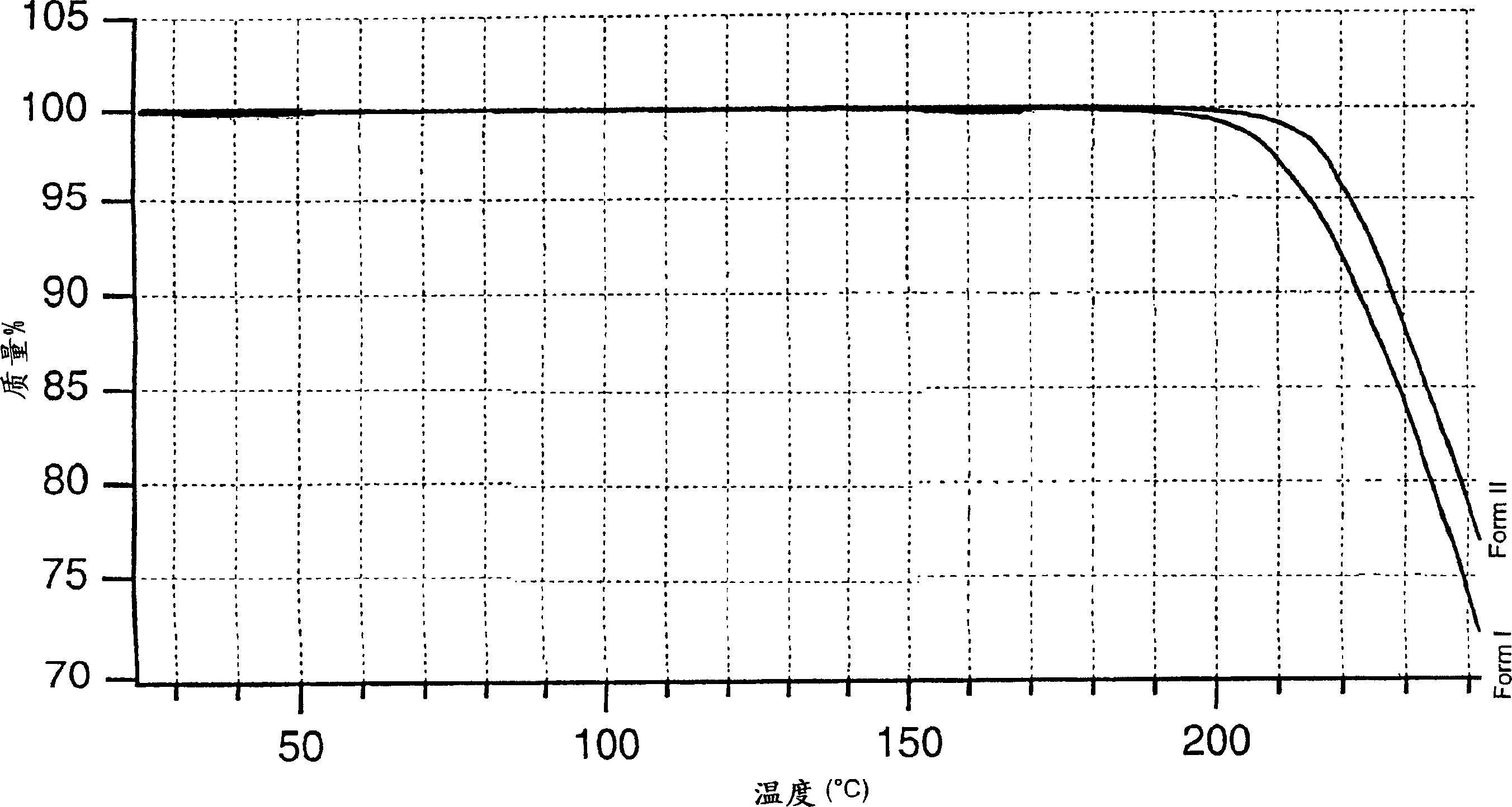
[0159] The organic phase obtained in Example 1 was then azeotropically distilled under a vacuum of about 250 mmHg at a temperature not exceeding 60°C. After removing about 50 ml of water, the solution was concentrated under the same temperature and pressure conditions to about 1 / 3 of the original volume and then neoethyl acetate was added to the original volume until the K.F. value (Karl Fisher value) was about 0.10-0.15% . The final suspension was cooled to 0-5°C. The solid was filtered, suspended in ethyl acetate (350 g), and stirred at 60-65°C for 1 hour. The bulk was cooled to 5-10°C and then filtered. The solid was dried in a 70°C oven. 133 g of dry crude lercanidipine hydrochloride (A) was obtained (75% yield), with a DSC peak of 150-152°C.
Embodiment 3
[0160] Embodiment 3 thick lercanidipine hydrochloride shape (B)
[0161] The organic phase finally obtained in Example 1 was heated under reflux (70-75° C.) and the water contained in the solution was removed with a DeanStark apparatus (Spaziani Rolando, Nettuno, Rome, Italy) until a K.F. value of about 2% was obtained. Thereafter the whole is distilled at atmospheric pressure up to 3 / 4 of the initial volume. Neoethyl acetate was added to bring the solution up to its original volume. The K.F. value at the end of the treatment was 0.9-1.1%. The final solution was cooled to 0-5 °C. A solid slowly precipitated, which was filtered. The solid thus obtained was suspended in ethyl acetate (350 g) and stirred at 60-65°C for 1 hour. The whole was cooled to 5-10°C, then filtered and dried in an oven at 70°C to obtain 133 g of crude lercanidipine hydrochloride (B), with a DSC peak of 131-135°C; 75% yield.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com