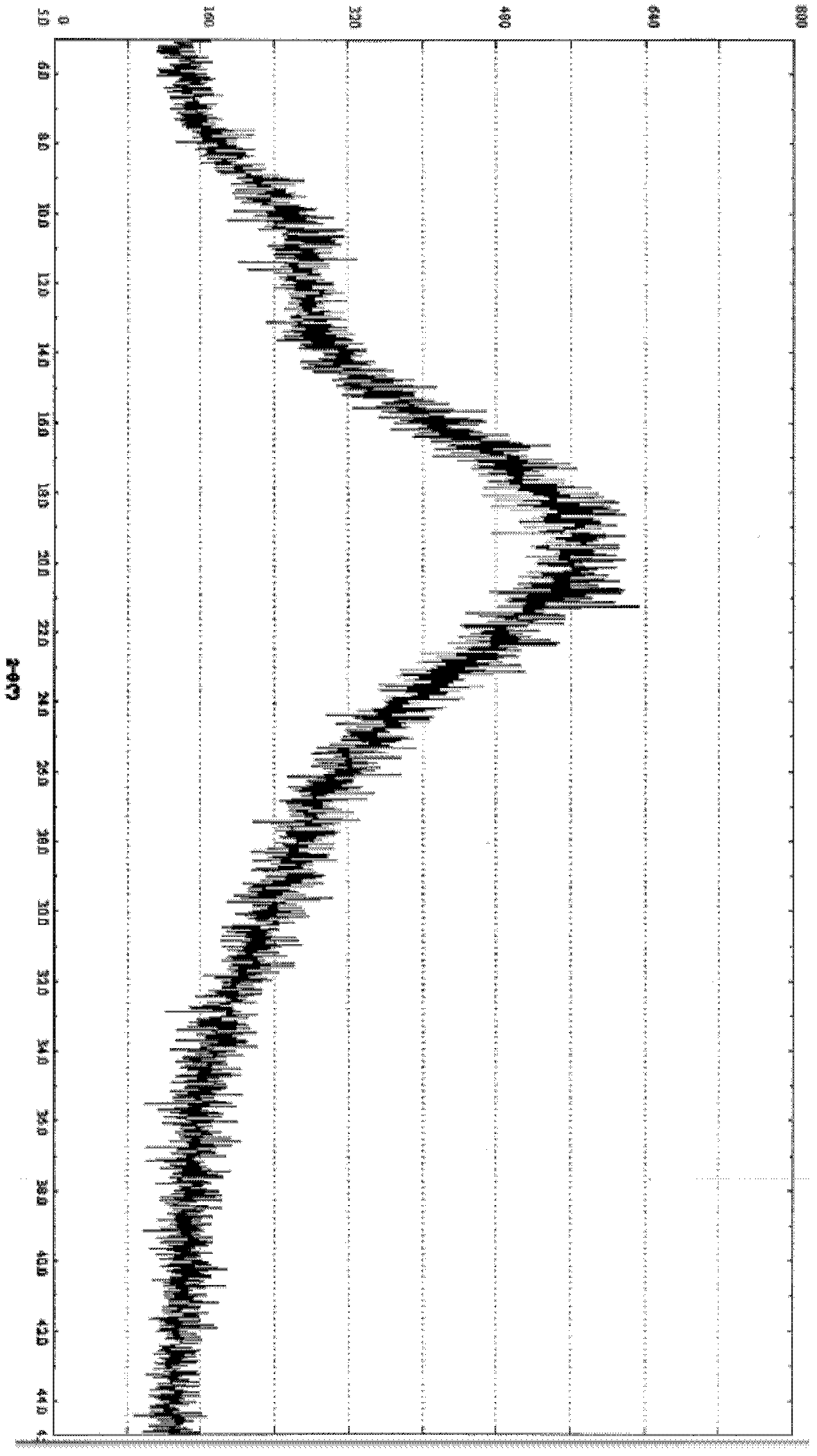
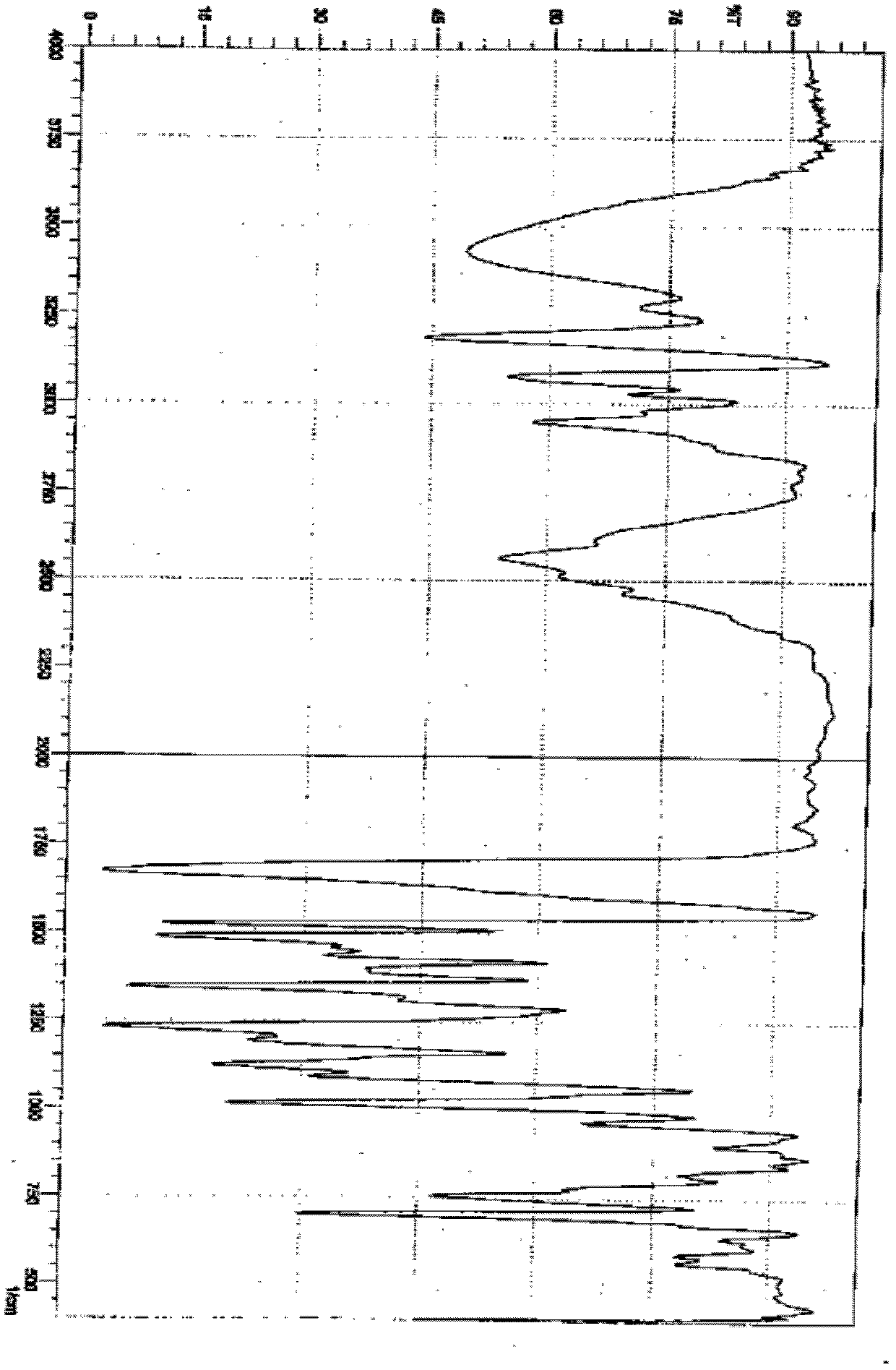
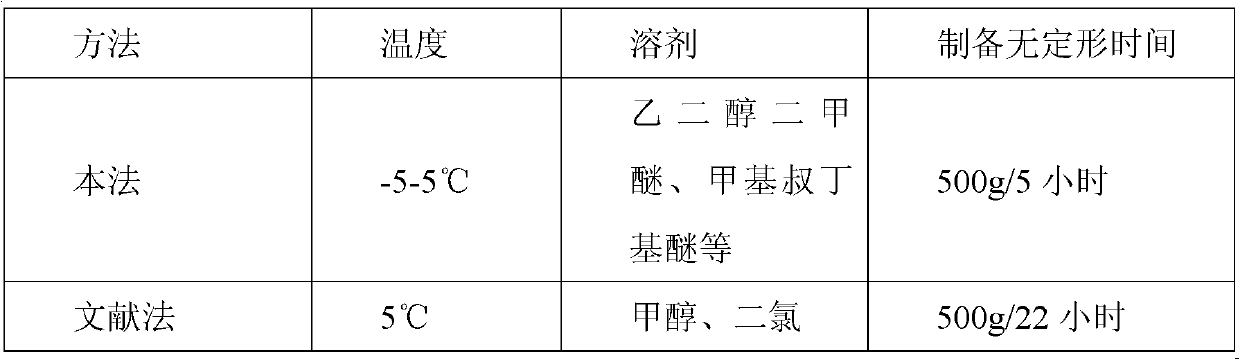
Amorphous lercanidipine hydrochloride and preparation method thereof
An amorphous technology of lercanidipine hydrochloride, which is applied in the field of high-purity amorphous lercanidipine hydrochloride and its preparation, can solve the problems of unsuitability for industrial production, cumbersome operation, and long cycle, and achieve easy commercial industrial scale implementation , high purity, short cycle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: Preparation of free state lercanidipine base solution
[0050] Add 20g of crude lercanidipine hydrochloride into 100mL of dichloromethane, adjust the pH to 7.5-8.0 with saturated aqueous sodium bicarbonate solution at 5°C under stirring, leave to stand for layers, and separate the organic layer to obtain free lercanidipine The base dichloromethane solution is set aside.
Embodiment 2
[0051] Embodiment 2: the preparation of lercanidipine oxalate crude product
[0052] Add 30ml of an aqueous solution of 2.9g oxalic acid to the lercanidipine base dichloromethane solution prepared in Example 1, stir well and separate the organic phase, dry it over anhydrous sodium sulfate, vacuum filter and evaporate to dryness to obtain Lercani Crude dipine oxalate (yield = 18.6 g, HPLC purity: 98.6%).
Embodiment 3
[0053] Embodiment 3: primary crystallization of lercanidipine oxalate
[0054] Dissolve 10g of crude lercanidipine oxalate in 50ml of ethanol at 80°C under stirring in a dry reaction bottle, slowly cool to 20°C, stir at a medium speed for 8 hours, a pale yellow solid precipitates, filter, and wash twice with 10ml of ethanol , air-dried at 45° C. to obtain a light yellow solid (yield = 7.6 g, HPLC purity: 99.6%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com