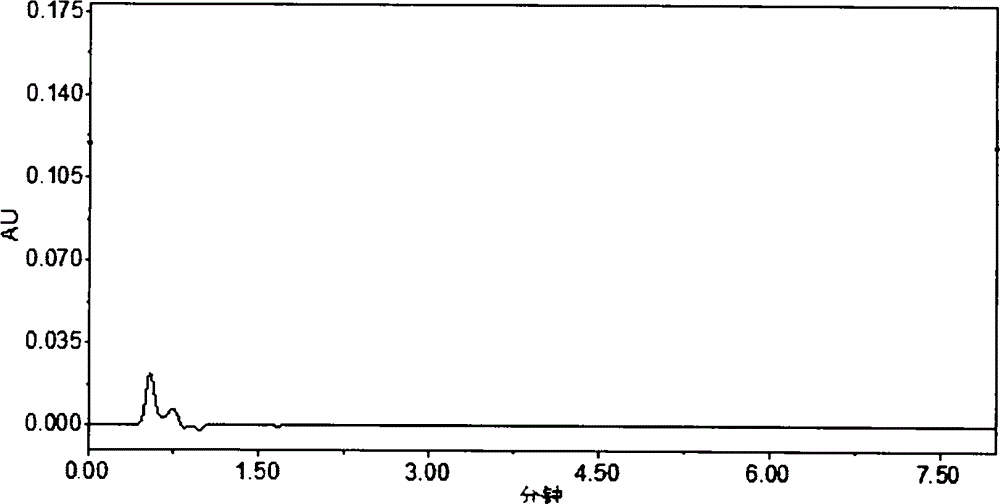
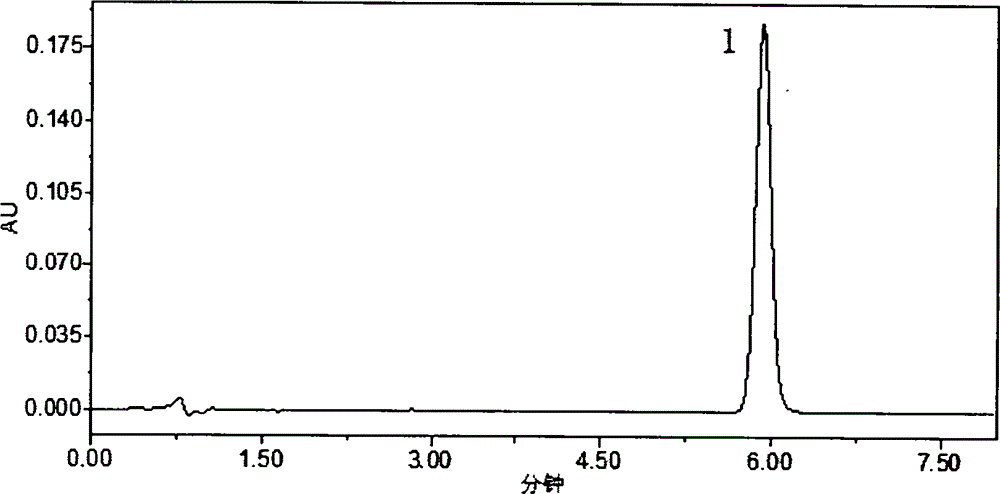
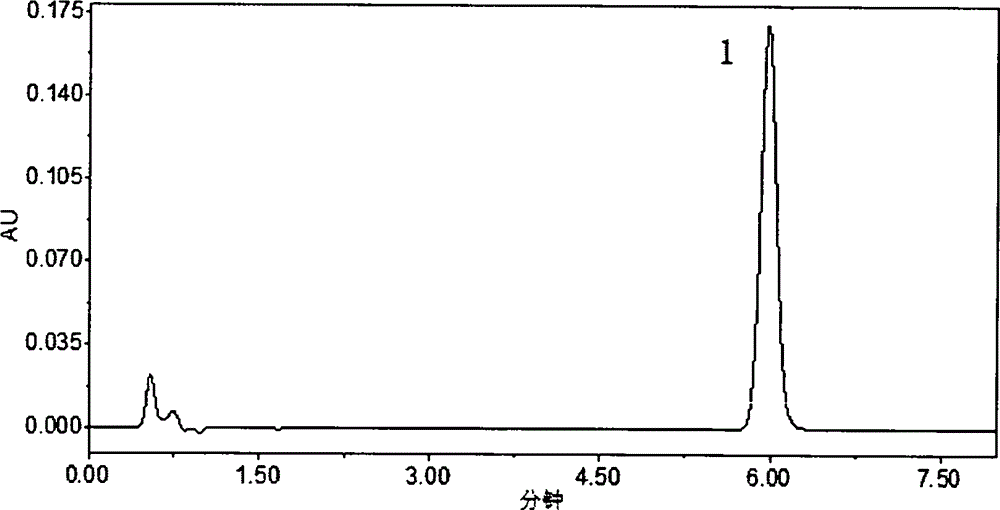
HPLC (High Performance Liquid Chromatography) method for determining dissolution rate of Lercanidipine hydrochloride tablet
A technology of lercanidipine hydrochloride and lerca hydrochloride, which can be used in measuring devices, instruments, scientific instruments, etc., and can solve the problems of excipients that interfere with specificity and lack of strength.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] 1 Instruments and reagents
[0016] Waters 2695 high-performance liquid chromatograph; Swiss SOTAXAT7smart dissolution apparatus (Swiss SOTAX company), FODT-601 optical fiber drug dissolution real-time tester (Xinjiang Fukesi Analytical Instrument Co., Ltd.). Both lercanidipine hydrochloride tablets (10 mg / tablet specification, batch numbers 110901, 111001, 111002) and lercanidipine hydrochloride working reference substance (99.7% content) were provided by Chongqing Shenghuaxi Pharmaceutical Co., Ltd. Acetonitrile was chromatographically pure (Shandong Yuwang Industrial Corporation Chemical Plant), and other reagents were analytically pure.
[0017] 2 Methods and results
[0018] 2.1 Chromatographic conditions
[0019] The column is Waters SunFire C 18 (4.6mm×150mm, 5μm); the mobile phase is 0.15mol L -1 Sodium perchlorate solution (adjust the pH value to 3.0 with 70% perchloric acid)-acetonitrile (40:60); column temperature 25°C; detection wavelength 240nm; flow ra...
Embodiment 2
[0046] 2.1 Chromatographic conditions
[0047] The column is Waters SunFire C 18 (4.6mm×150mm, 5μm); the mobile phase is 0.15mol L -1 Sodium perchlorate solution (adjust the pH value to 4.0 with 70% perchloric acid)-acetonitrile (40:60); column temperature 25°C; detection wavelength 240nm; flow rate 1.0ml·min -1 ; The injection volume is 40 μL. The number of theoretical plates calculated based on the peak of lercanidipine hydrochloride is not less than 6000.
[0048] 4 Sample dissolution test
[0049] Get 6 tablets of this product, according to the dissolution test method (Chinese Pharmacopoeia 2010 edition two appendix XC dissolution test method), with 0.1mol L containing 0.3% (w / v) Tween 80 -1 500mL of hydrochloric acid solution is used as the dissolution medium, and the rotation speed is 50r·min -1 , operate according to the law, after 45 minutes, take the solution and filter it with a 0.45 μm microporous membrane as the test solution; take another appropriate amount o...
Embodiment 3
[0054] 2.1 Chromatographic conditions
[0055] The column is Waters SunFire C 18 (4.6mm×150mm, 5μm); the mobile phase is 0.15mol L -1 Sodium perchlorate solution (adjust the pH value to 3.0 with 70% perchloric acid)-acetonitrile (40:60); column temperature 30°C; detection wavelength 240nm; flow rate 1.0ml·min -1 ; The injection volume is 40 μL. The number of theoretical plates calculated based on the peak of lercanidipine hydrochloride is not less than 6000.
[0056] 4 Sample dissolution test
[0057] Get 6 tablets of this product, according to the dissolution test method (Chinese Pharmacopoeia 2010 edition two appendix XC dissolution test method), with 0.1mol L containing 0.3% (w / v) Tween 80 -1 500mL of hydrochloric acid solution is used as the dissolution medium, and the rotation speed is 50r·min -1 , operate according to the law, after 45 minutes, take the solution and filter it with a 0.45 μm microporous membrane as the test solution; take another appropriate amount o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com