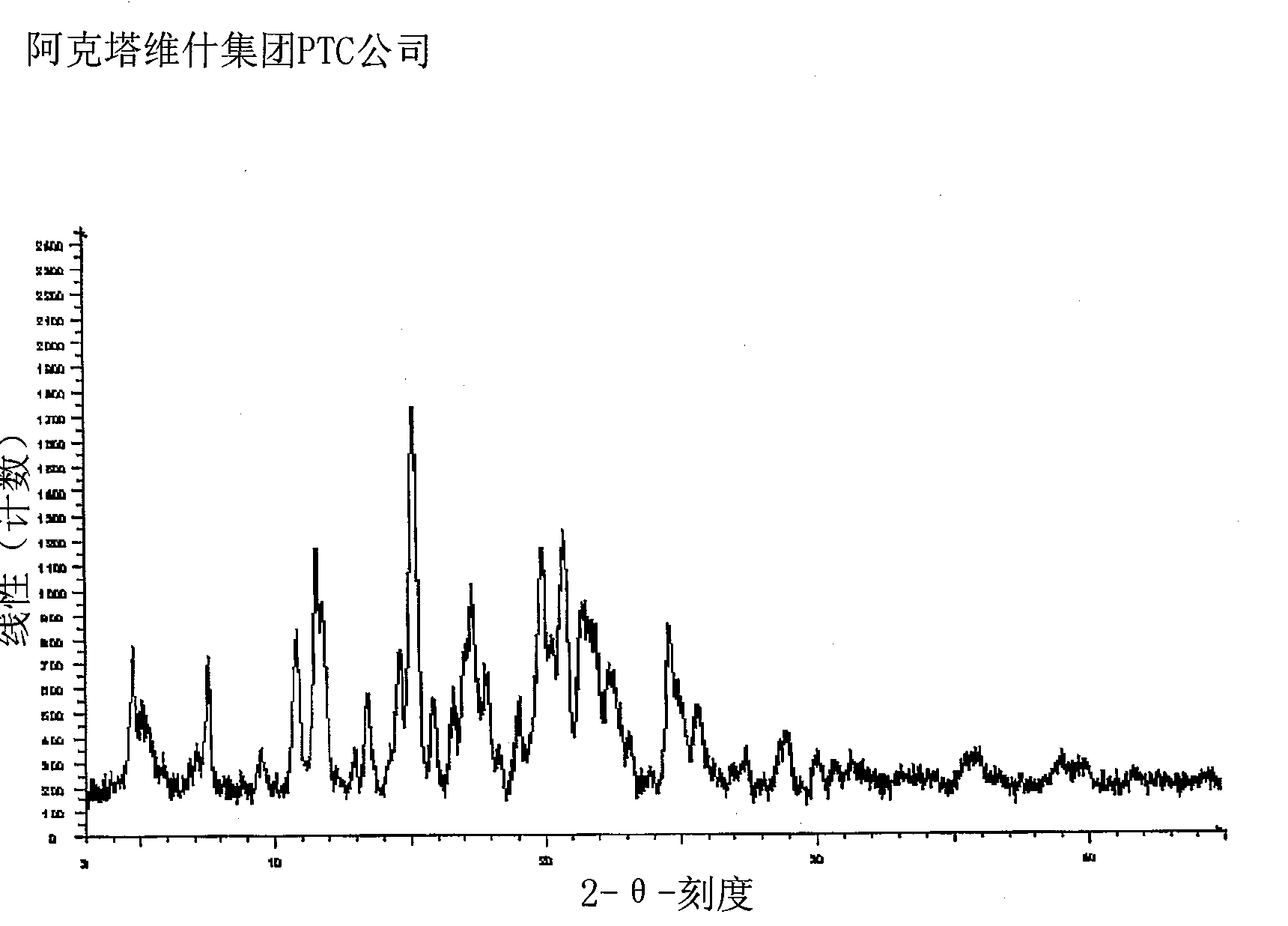
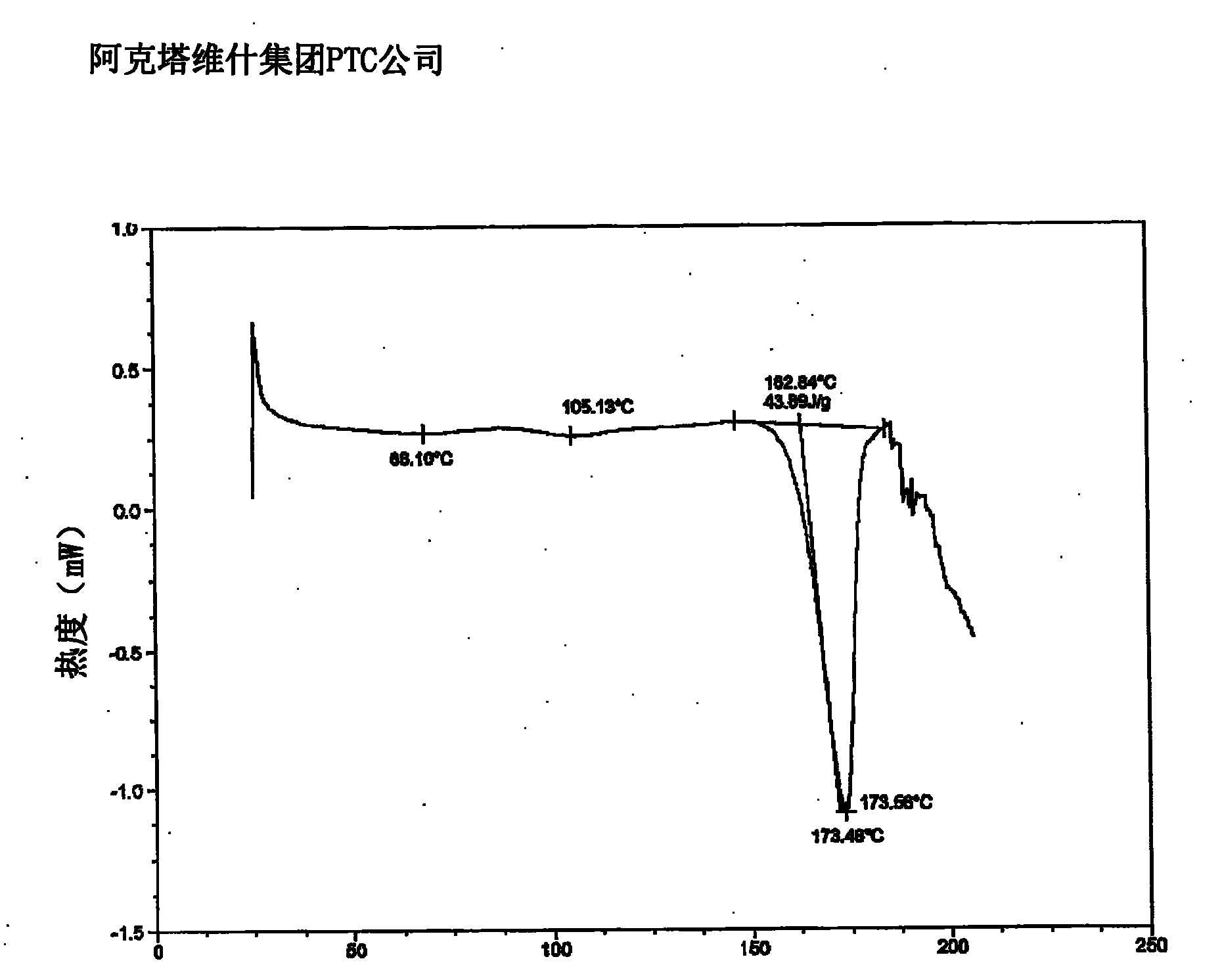
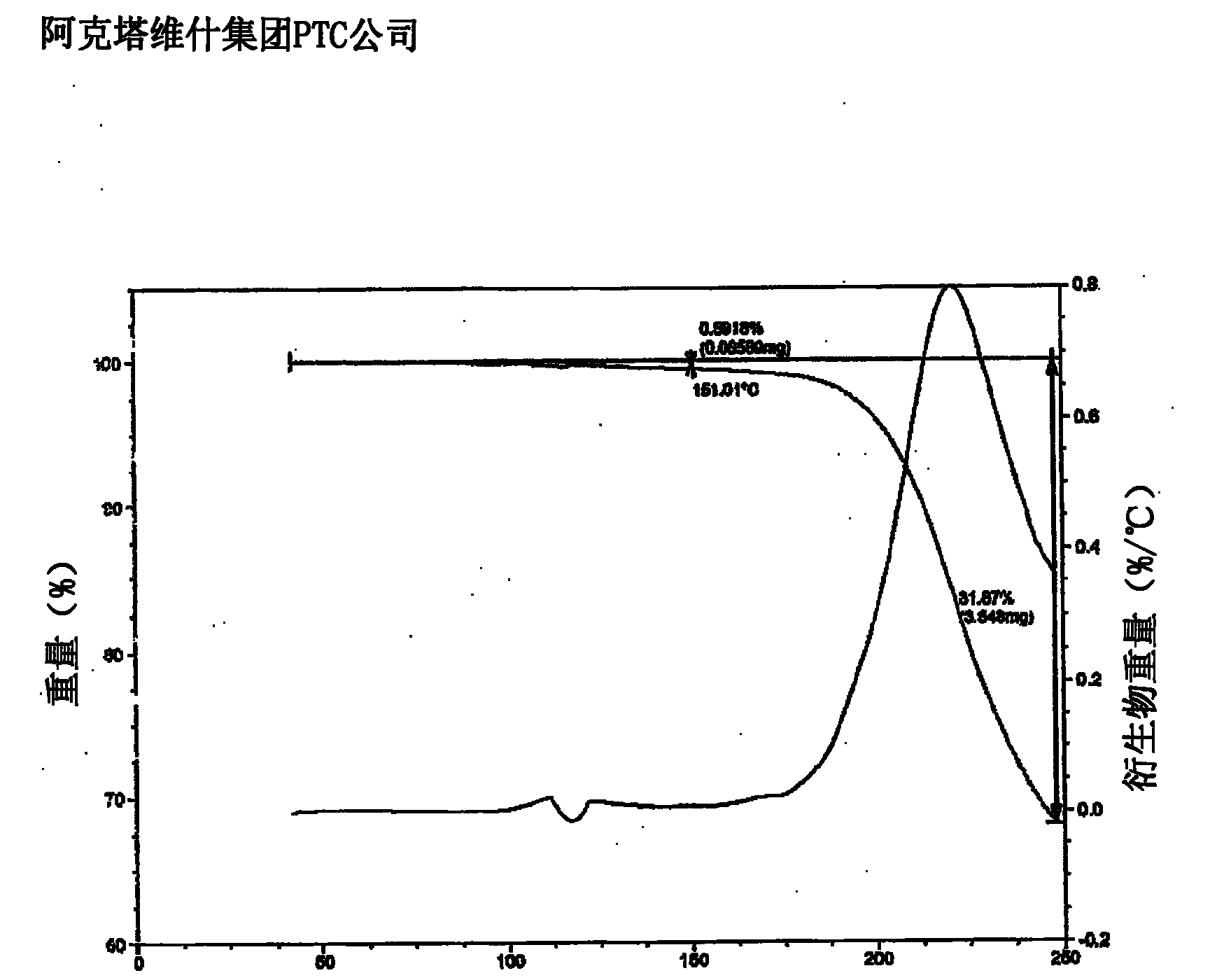
Lercanidipine hydrochloride polymorphs and an improved process for preparation of 1,1,N-trimethyl-N-(3,3-diphenylpropyl)-2-aminoethyl acetoacetate
A technology of ethyl aminoacetoacetate and lercanidipine hydrochloride, which is applied in the preparation of amorphous lercanidipine hydrochloride, new crystalline lercanidipine hydrochloride and its preparation field, can solve problems such as difficulty in implementation, and achieve low risk Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] According to another aspect of the present invention, a method for preparing lercanidipine hydrochloride crystal form Y is provided, the method comprising:
[0070] a) providing a solution of lercanidipine hydrochloride in an amide solvent;
[0071] b) adding an aliphatic ester solvent to the solution; and
[0072] c) recovering substantially pure lercanidipine hydrochloride Form Y from the solution.
[0073] Exemplary amide solvents include, but are not limited to, N,N-dimethylacetamide, N,N-diethylacetamide, N,N-dimethylacetoacetamide, N,N-diethylacetoacetamide , formanilide, N-methylformanilide, N, N-di-n-propylacetamide, N, N-diisopropylacetamide, di-n-butylacetamide, N, N-dimethyl- 2,2-Diphenylacetamide and mixtures thereof. A more specific amide solvent is N,N-dimethylacetamide.
[0074] The step (a) of providing a solution of lercanidipine hydrochloride comprises dissolving any form of lercanidipine hydrochloride in an appropriate amide solvent, or obtaining ...
Embodiment 1
[0108] Preparation of 1,1,N-trimethyl-N-(3,3-diphenylpropyl)-2-aminoacetoacetate ethyl ester:
[0109] 2,N-Dimethyl-N-(3,3-diphenylpropyl)-1-amino-2-propanol (5 g) and methyl acetoacetate (7.5 g) were dissolved at 25-30 °C In xylene (50ml), zinc dust (1.75g) was then added. The reaction mixture was heated at 140-145°C for 7 hours. Next, xylene was distilled off while adding xylene to maintain the volume of xylene in the reaction mixture. The reaction mixture was cooled at 25-30°C, then the catalyst was removed by filtration. Distillation was performed under reduced pressure to remove xylene. The resulting residue was degassed for 1 hour to yield the title compound as a viscous brown oil.
Embodiment 2
[0111] Preparation of 1,1,N-trimethyl-N-(3,3-diphenylpropyl)-2-aminoethyl α-acetyl-3-nitrocinnamate hydrochloride:
[0112] A mixture of ethyl 1,1,N-trimethyl-N-(3,3-diphenylpropyl)-2-aminoacetoacetate (5 g) and 3-nitrobenzaldehyde (2 g) was dissolved in toluene (50ml). The solution was cooled to 0 °C, and dry hydrogen chloride gas was bubbled through the solution until the solution was saturated. The reaction mixture was stirred at 0°C for 9 hours. The organic layer was separated from the reaction mixture and washed with toluene (25ml). The resulting oily residue was dissolved in dichloromethane (100ml). The resulting solution was dried over calcium chloride, followed by distillation of dichloromethane at 30-35°C under reduced pressure to yield 5.5 g of 1,1,N-trimethyl-N-(3,3-diphenylpropyl)- 2-Aminoethyl alpha-acetyl-3-nitrocinnamate hydrochloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com