Lercanidipine hydrochloride crystal and preparation method thereof
A technology of lercanidipine hydrochloride and crystals, which is applied in the field of crystals of new forms of lercanidipine hydrochloride and its preparation, and can solve the problems of increasing the difficulty and cost of large-scale production, complicated preparation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of lercanidipine hydrochloride hydrate
[0038] Take 60 g of the above-mentioned lercanidipine base raw material, dissolve it with 5 times the volume of dichloromethane (or dichloroethane, chloroform), add 5 to 10 times the volume of 10% (w) hydrochloric acid aqueous solution, fully stir and let it stand , separate the water layer, wash the dichloromethane phase with saturated brine, and recover the solvent by distillation or vacuum distillation, add 10 times the volume of water to the residue, stir at room temperature to crystallize, suction filter, wash the solid with water, and dry at 70- Vacuum-dried at 80°C to obtain 50 g of lercanidipine hydrochloride hydrate, with a moisture content of 4.5%, a melting temperature of 110-120°C, and an HPLC purity of ≥95%.
Embodiment 2
[0039] Example 2 Preparation of lercanidipine hydrochloride Form (HX) crystals
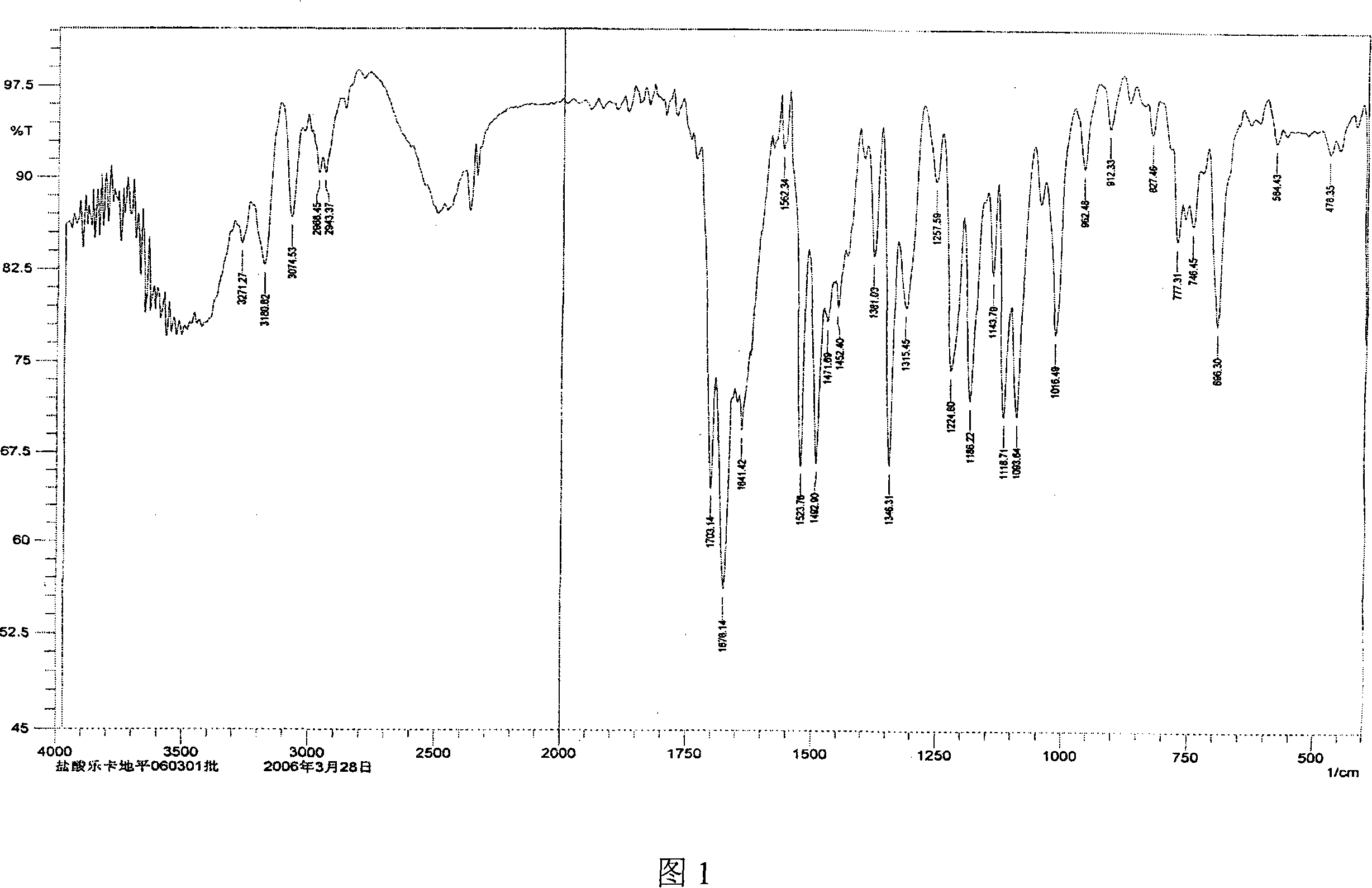
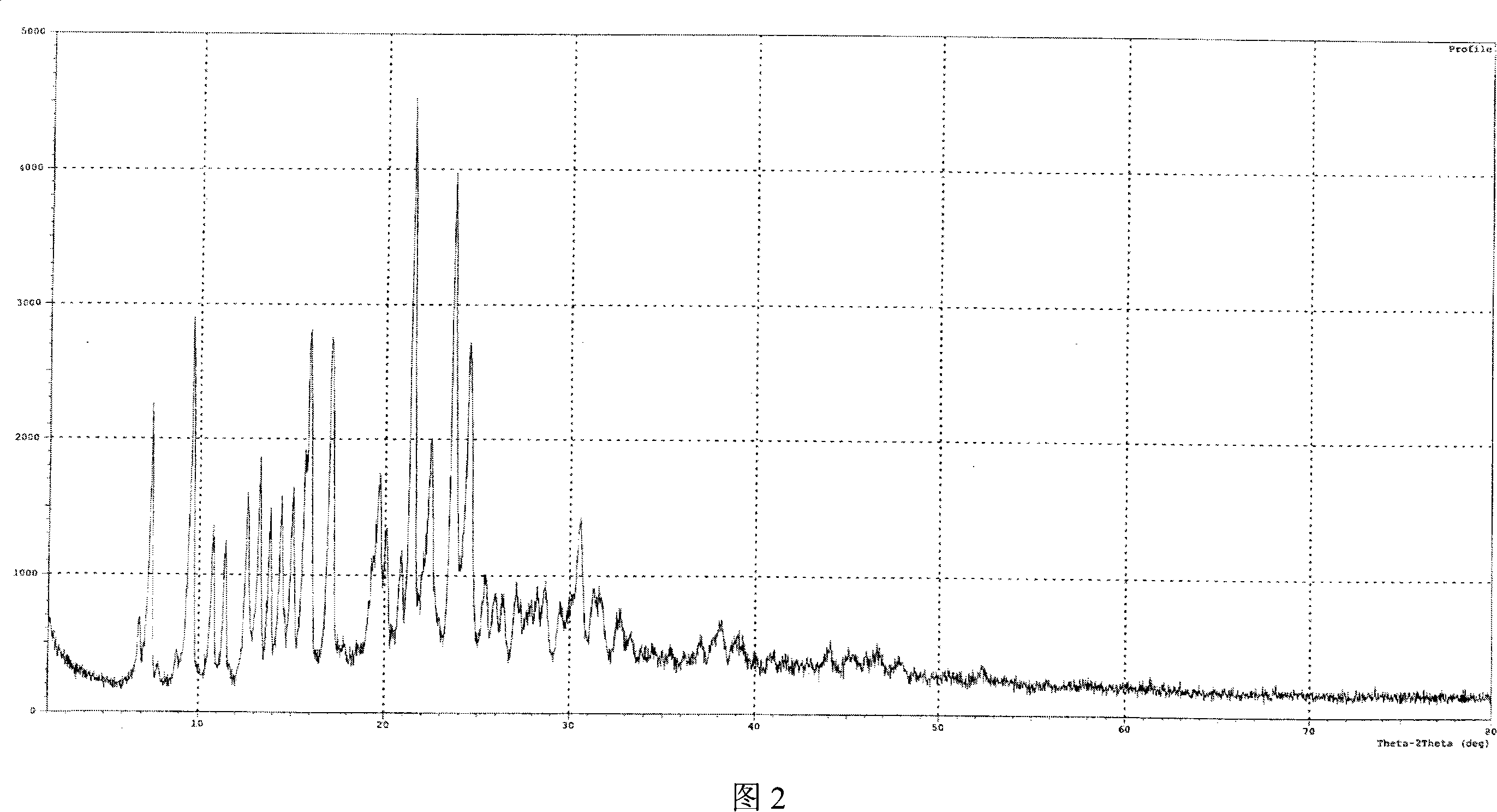
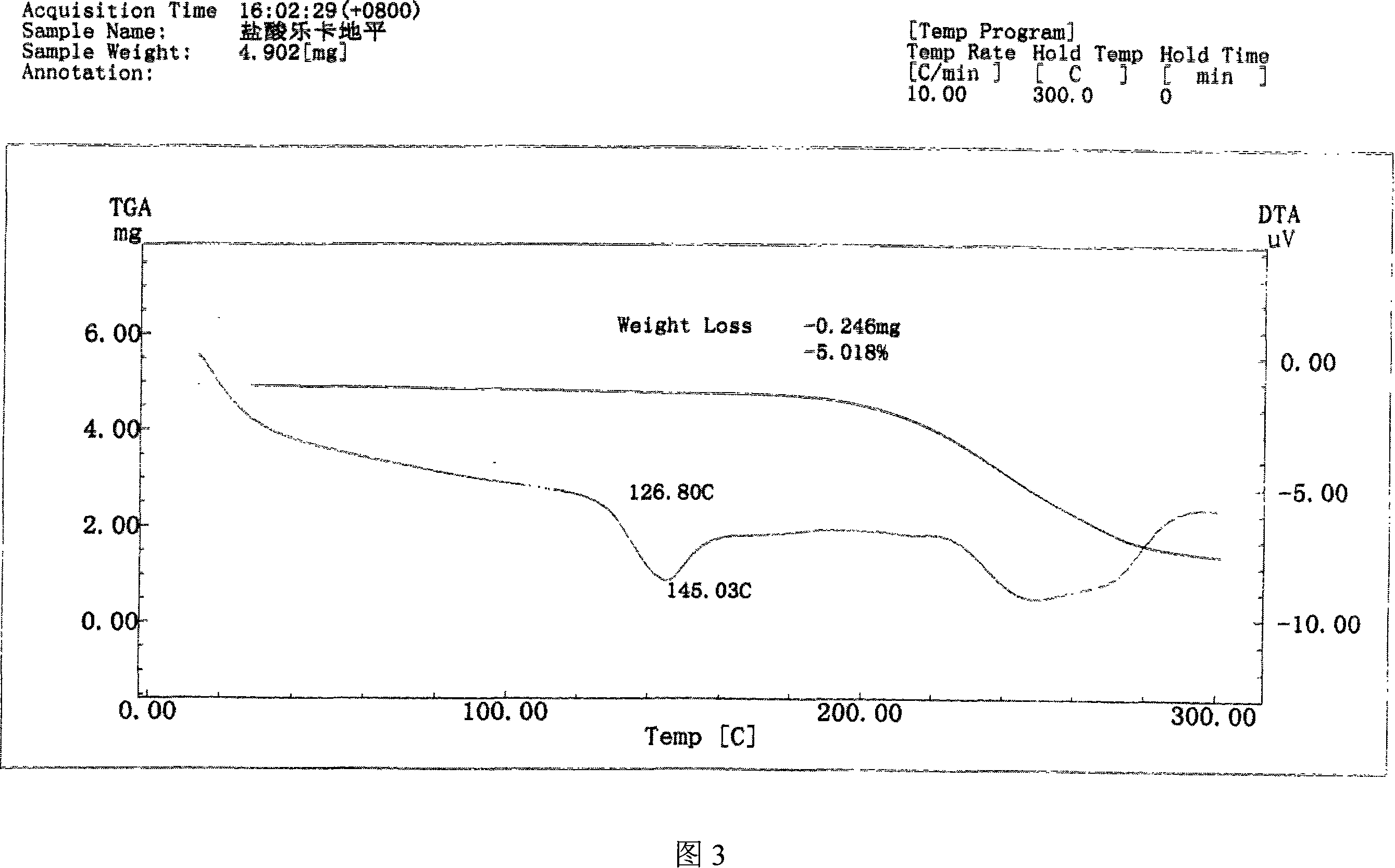
[0040]Get 10 g of lercanidipine hydrochloride hydrate obtained in Example 1, add 30 ml of DMF, stir to dissolve completely, then add 200 ml of ethyl acetate dropwise to the solution, then cool the solution to 0~5 ° C, continue stirring until the solid is fully separated , filtered with suction, and the filter cake was vacuum-dried at 70-80°C for 8 hours to obtain 7 g of anhydrous lercanidipine hydrochloride Form (HX) crystals, with HPLC purity ≥ 99%, and a melting temperature of 125°C-142°C. The IR spectrum, X-ray diffraction spectrum and thermal analysis spectrum (DSC, TG diagram) of the product are shown in Figure 1, Figure 2 and Figure 3 respectively.
Embodiment 3
[0041] Example 3 Preparation of lercanidipine hydrochloride Form (HX) crystals
[0042] Get the lercanidipine hydrochloride hydrate 10g of embodiment 1, add 40ml DMSO, after stirring to dissolve completely, add dropwise 200ml isopropyl acetate in the solution, then continue to stir at normal temperature until a large amount of solids are precipitated, suction filtration, filter cake Vacuum-dried at 70-80°C for 8 hours to obtain 6 g of anhydrous lercanidipine hydrochloride Form (HX) crystals, with HPLC purity ≥ 99% and melting temperature of 130°C-141°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com