Crystal form of lercanidipine hydrochloride and preparation method thereof and crystal form-containing medicinal composition
A technology of lorca hydrochloride and dipine crystals, which is applied in the field of new crystal forms and preparation methods thereof and pharmaceutical compositions containing the crystal forms, can solve the problems of complex preparation methods and the like, and achieve high purity, good stability and simple method. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 Preparation of lercanidipine hydrochloride raw material
[0039] The raw material of lercanidipine hydrochloride is according to the method of US4705797, with N-methyl-N-(3,3-diphenylpropyl)-1-amino-2-propanol and diketene as raw materials, after esterification and condensation, and The crude lercanidipine in the form of oil was prepared by cyclization of methyl β-aminobutyrylate. After purification, the purity analysis by HPLC was 85.0%-99.5%, which was used as a raw material for subsequent preparation.
Embodiment 2
[0040] Embodiment 2 Preparation of lercanidipine hydrochloride crystal form
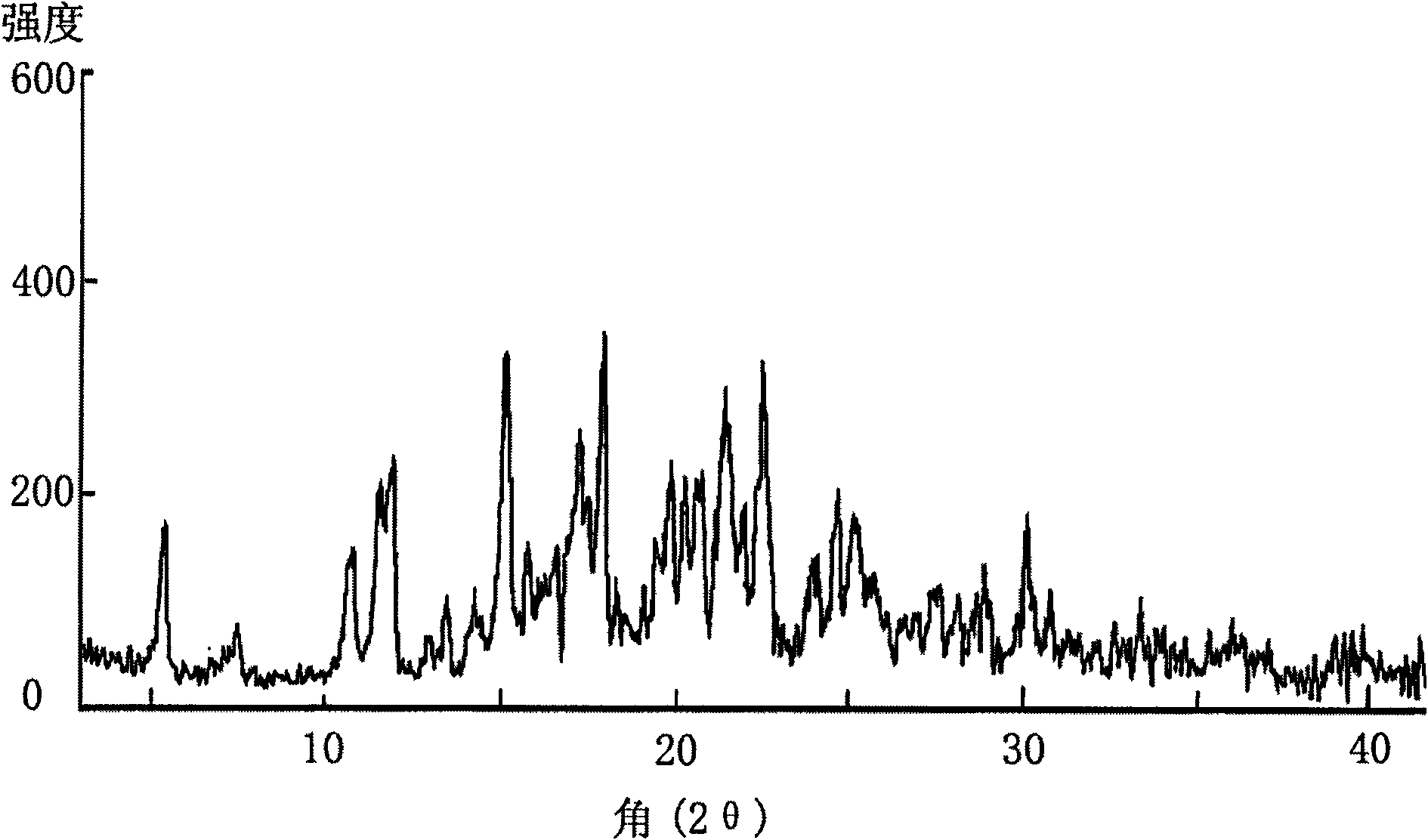
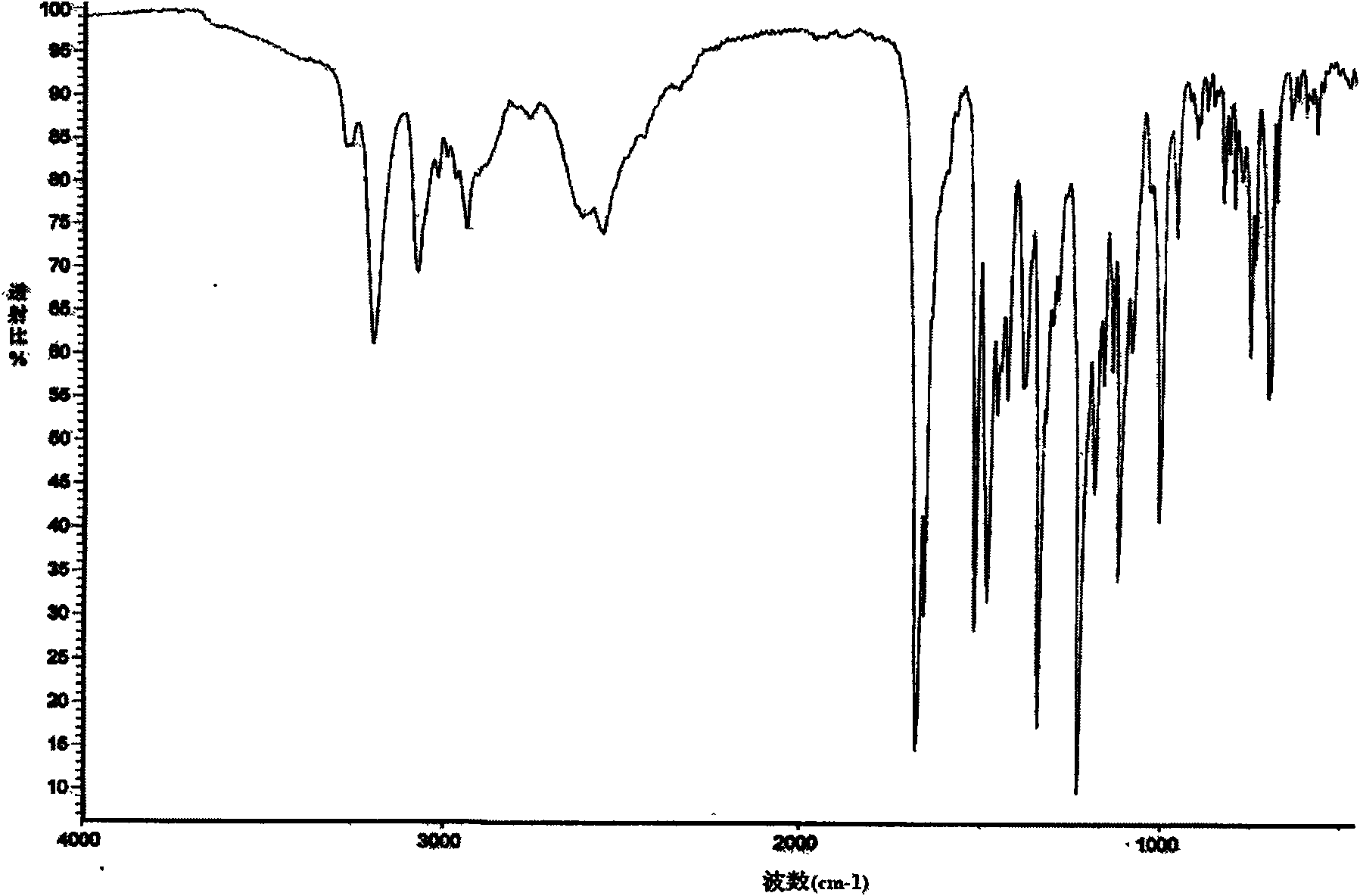
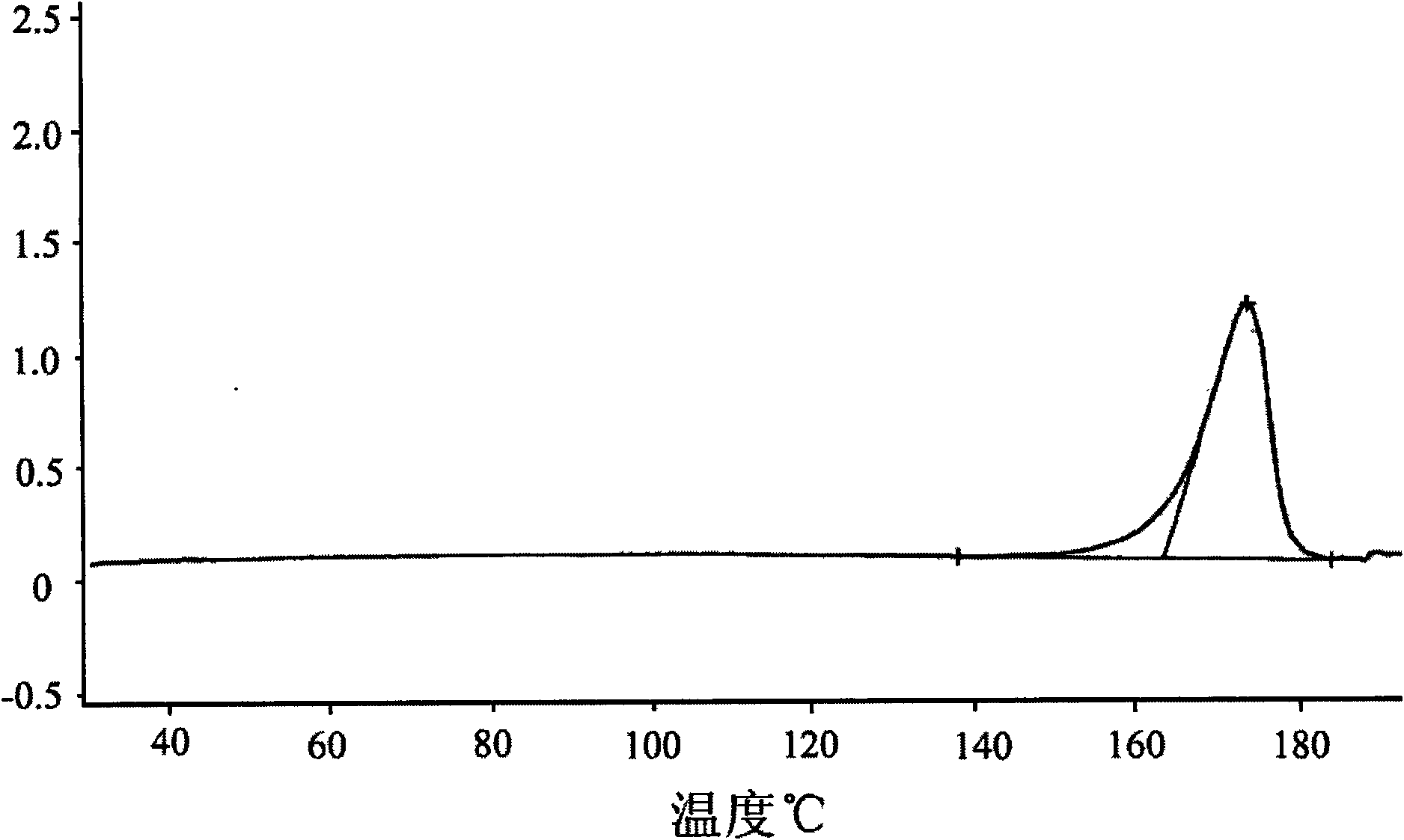
[0041]10g of lercanidipine hydrochloride with a purity of 99.5% was dissolved in methanol, evaporated to dryness until oily, added acetone: isopropyl acetate = 1:2, 500ml, stirred and dissolved, kept refrigerated at 0-5°C, pumped after 12 hours filtered, and vacuum dried at 70°C for 24 hours to obtain 8.8 g of the new crystalline product with a purity of 99.9%. The X-ray diffraction spectrum of gained new crystal form product, infrared spectrum (IR) collection of illustrative plates and differential scanning calorimetry (DSC) collection of illustrative plates are respectively as follows figure 1 , figure 2 with image 3 shown.
Embodiment 3
[0042] Embodiment 3 Preparation of lercanidipine hydrochloride crystal form
[0043] 10g of lercanidipine hydrochloride oily matter with a purity of 85% was added with acetone: isopropyl acetate=1: 30, 50ml, after stirring to dissolve, the new crystal seed crystal obtained as in Example 2 was added, and after stirring at room temperature for 12 hours Suction filtration and vacuum drying at 70°C for 24 hours gave 8.3 g of the new crystalline product with a purity of 99.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com