Complex formulation comprising lercanidipine hydrochloride and valsartan and method for the preparation thereof
A technology of lercanidipine hydrochloride and lercadipine hydrochloride, applied in medical preparations containing active ingredients, pill delivery, drug combination, etc., can solve side effects and other problems, achieve the effect of reducing side effects and increasing dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
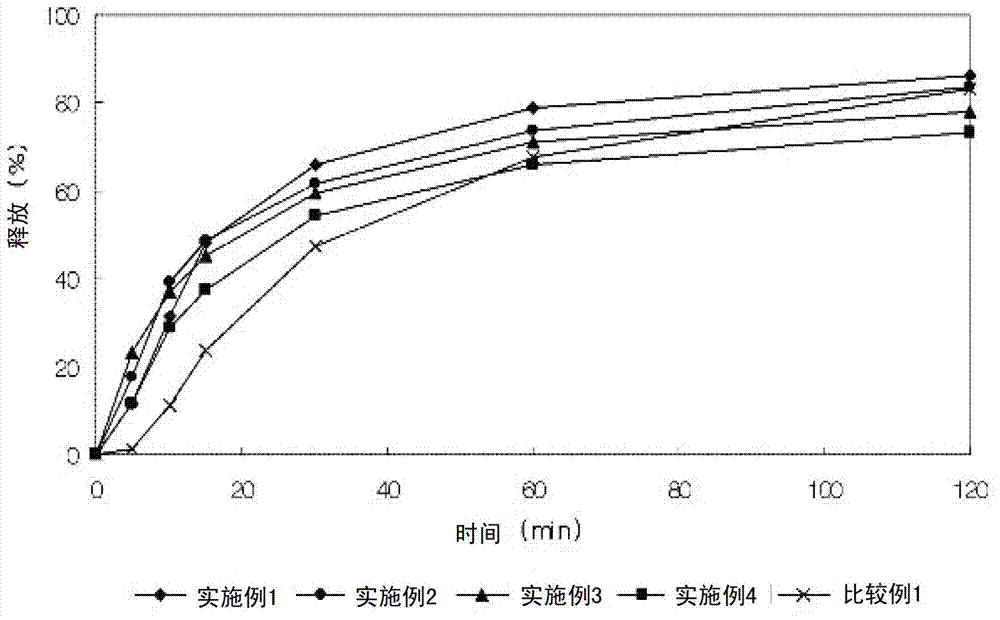
Embodiment 1-4
[0053] Examples 1-4: Preparation of isolated particles
[0054] [Table 1]
[0055]
[0056] The lercanidipine hydrochloride granules and valsartan granules having the above composition were wet granulated with distilled water, dried and ground into 25 meshes. The two granules are mixed and compressed into a composite tablet, which is then film-coated. Selling Colorcon as PVP II was used as a film coating material, and film coating was performed in the same manner in all of the following Examples and Comparative Examples. The mixture was mixed for 5 minutes or longer to fully wet the super-integrating agent added during wet granulation. Such intensive mixing time increases the porosity of the granules, aids disintegration time to prevent gelling. In the examples below, wet granulation was performed as described above.
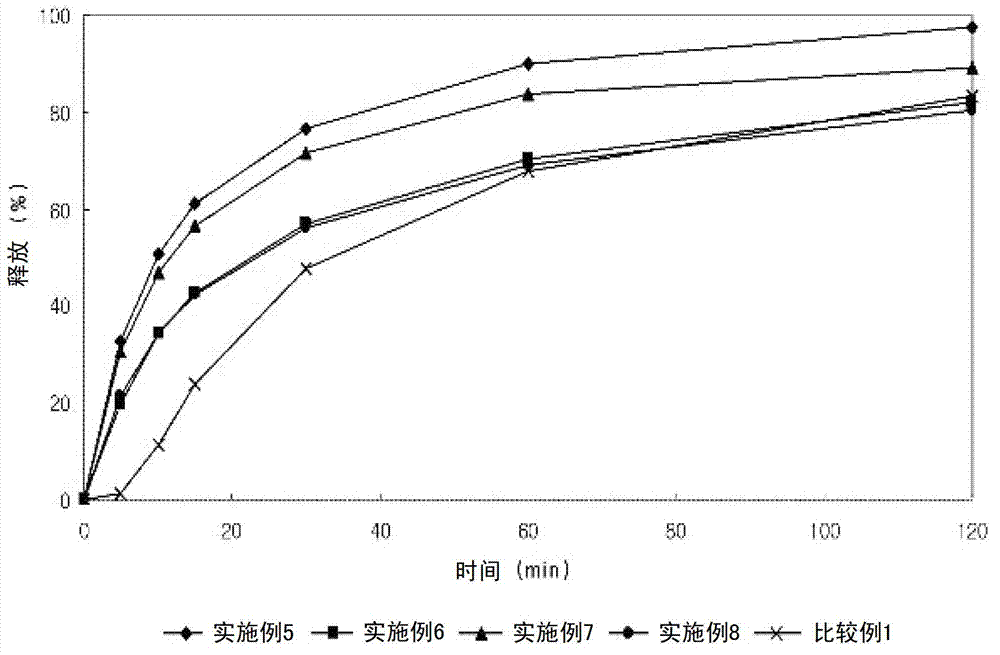
Embodiment 5-8
[0057] Embodiment 5-8: Preparation of composite bilayer tablet
[0058] [Table 2]
[0059]
[0060] The lercanidipine hydrochloride granules and valsartan granules having the above composition were wet granulated with distilled water, dried and ground into 25 meshes. The two sets of granules were compressed into a bilayer solid form, whereby the bilayer solid form was film coated.
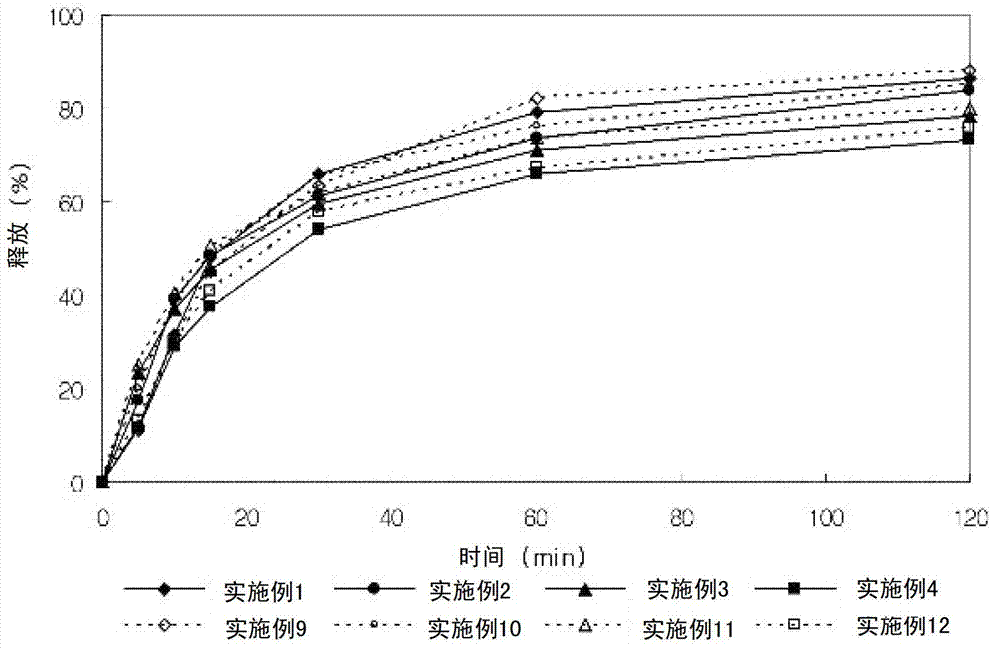
Embodiment 9-12
[0061] Embodiment 9-12: Preparation of composite three-layer tablet
[0062] [table 3]
[0063]
[0064] The lercanidipine hydrochloride granules and valsartan granules having the above composition were wet granulated with distilled water, dried and ground into 25 meshes. Valsartan granules were added to make the first tablet portion, a barrier layer was added to make the second tablet portion, and lercanidipine hydrochloride granules were added to make the third tablet portion. Composite three-layer tablets are obtained and film-coated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com