Lercanidipine hydrochloride sustained release preparation and preparation method thereof
A technology of lercanidipine hydrochloride and lerca hydrochloride, which can be used in pharmaceutical formulations, drug delivery, cardiovascular system diseases, etc., and can solve problems such as difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
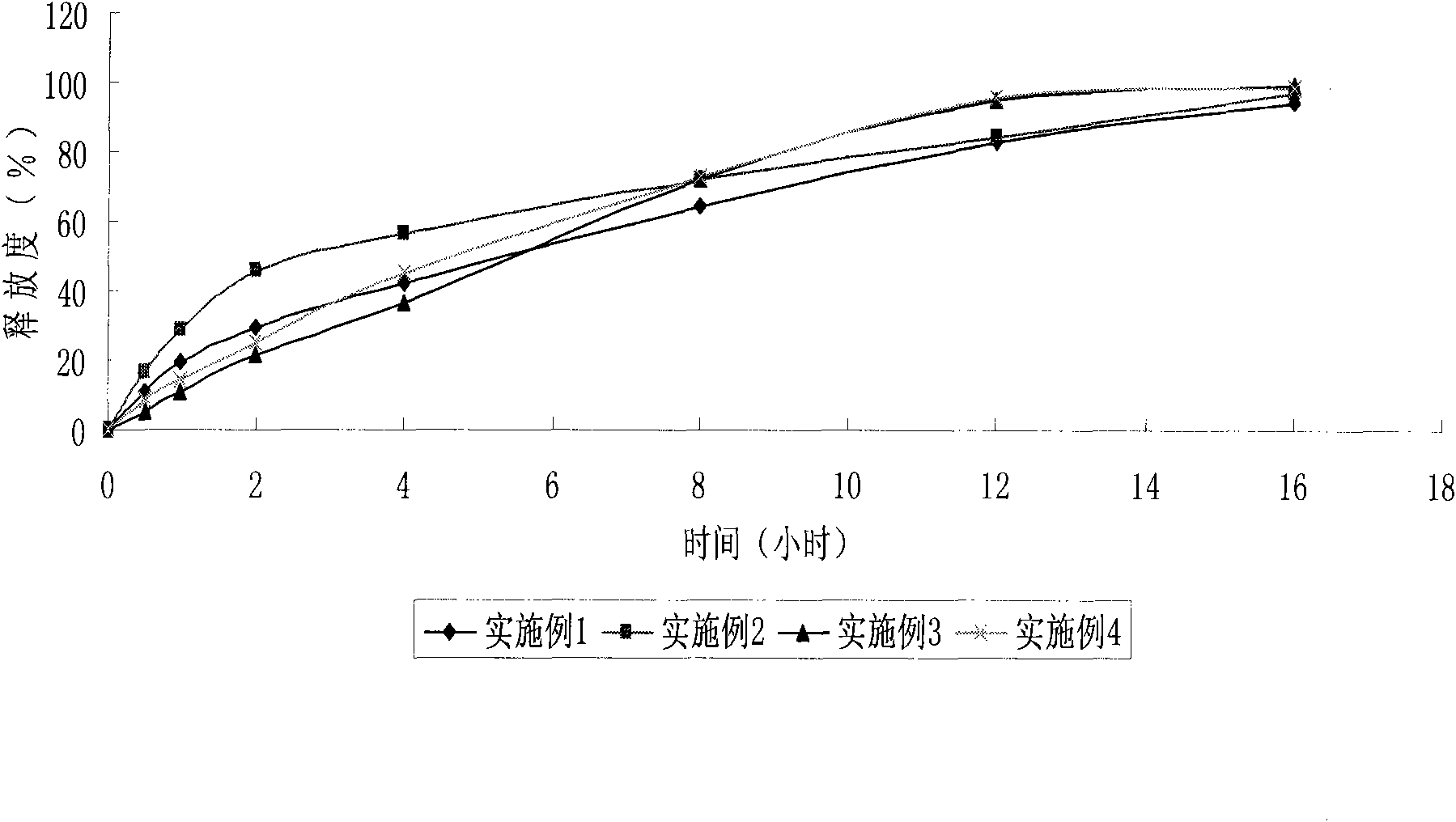
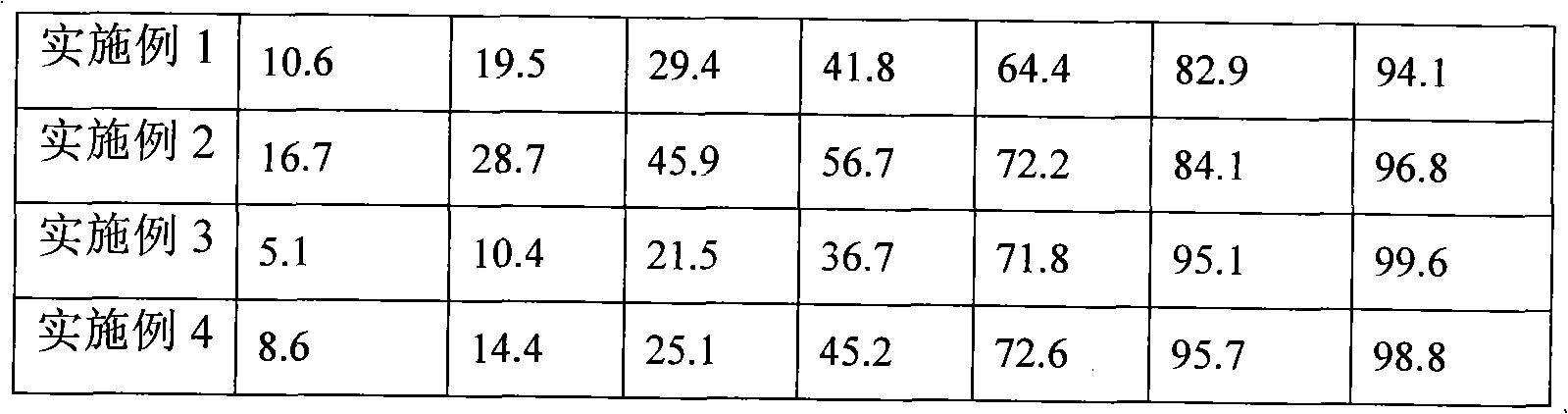
Examples
Embodiment 1
[0036] Plain Tablet Prescription:
[0037] Lercanidipine Hydrochloride 10g
[0038] Copovidone 20g
[0039] Microcrystalline Cellulose 60g
[0040] Polyethylene glycol 15g
[0041] Hypromellose 80g
[0043] Proper amount of ethanol
[0044] Coating Solution Prescription:
[0045] Opadry (Red) 40g
[0046] Appropriate amount of water
[0047]
[0048] Makes 1000 pieces
[0049] Preparation process
[0050] 1 Preparation process of plain tablets
[0051] 1.1 Preparation of dispersion Take the prescribed amount of lercanidipine hydrochloride and copovidone and dissolve them in ethanol to make a 5% (w / w) copovidone ethanol dispersion for subsequent use;
[0052] 1.2 Granulation Put hydroxypropyl methylcellulose, microcrystalline cellulose and polyethylene glycol together into a mixing granulator, mix them, and add copovidone ethanol dispersion for wet granulation after uniformity, take out the granules with a 20...
Embodiment 2
[0060] Plain Tablet Prescription:
[0061] Lercanidipine Hydrochloride 10g
[0062] Ethyl cellulose 80g
[0063] Microcrystalline Cellulose 20g
[0064] Lactose 60g
[0065] Povidone 40g
[0066] Stearic acid 4g
[0067] Proper amount of ethanol
[0068] Coating Solution Prescription:
[0069] Opadry 50g
[0070] Appropriate amount of water
[0071]
[0072] Makes 1000 pieces
[0073] Preparation process
[0074] 1 Preparation process of plain tablets
[0075] 1.1 Preparation of adhesive: Dissolve the povidone of the prescribed amount in ethanol to make a 10% (w / w) adhesive for subsequent use;
[0076] 1.2 Granulation: Sieve lercanidipine hydrochloride and lactose through 80-mesh sieve respectively, put them into a mixing granulator together with ethyl cellulose and microcrystalline cellulose, mix them, and add a binder for wet granulation after uniformity, take out The granules were wet sized with a 20-mesh sieve. After drying, pas...
Embodiment 3
[0082] Plain Tablet Prescription:
[0083] Lercanidipine Hydrochloride 20g
[0084] Carnauba Wax 80g
[0085] Microcrystalline Cellulose 50g
[0086] Sucrose 20g
[0087] Povidone 30g
[0089] Proper amount of ethanol
[0090] Makes 1000 pieces
[0091] Coating solution prescription: (1000 tablets dosage)
[0092] Cellulose diacetate 50g
[0093] Polyethylene glycol 6g
[0094] Dibutyl sebacate 4g
[0095] Proper amount of acetone
[0096]
[0097] Makes 1000 pieces
[0098] Preparation process
[0099] 1 Preparation process of plain tablets
[0100] 1.1 Preparation of adhesive: Dissolve the povidone of the prescribed amount in ethanol to make a 5% (w / w) adhesive for subsequent use;
[0101] 1.2 Granulation: After sieving sucrose and microcrystalline cellulose with 80 meshes respectively, put them into the mixing granulator together with lercanidipine hydrochloride and carnauba wax, mix them, and then add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com