Lercanidipine hydrochloride and losartan potassium compound preparation and preparation method thereof
A technology for lercanidipine hydrochloride and dipine losartan, applied in the field of medicine, can solve problems such as unreported hypertension, achieve good clinical application prospects, reduce adverse reactions, and improve the effects of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the preparation of lercanidipine-losartan compound preparation of the present invention
[0025] Weighing lercanidipine hydrochloride bulk drug and losartan bulk drug and sieving for standby; lercanidipine hydrochloride is 0.5-40%, and losartan potassium is 6.25-50%.
[0026] Weigh lactose-hydrate, microcrystalline cellulose, hydroxyethyl starch sodium (type A), povidone K30, magnesium stearate, pregelatinized starch, colloidal silicon dioxide and stir to mix evenly; 20-40%, microcrystalline cellulose 10-40%, hydroxyethyl starch sodium (type A) 10-20%, povidone K302-8%, magnesium stearate 0.5-2.5%, pregelatinized starch 5-15%, colloidal silicon dioxide 1-10%
[0027] Vacuum compression, milling, sieving and granulation; tableting to control tablet core hardness.
[0028] Preparation of isolation coat solution: Take Opadry, add it to ethanol under stirring condition, stir until dispersed, then add purified water and stir to make isolation coat solution. ...
Embodiment 2
[0032] Embodiment 2: Preparation of lercanidipine-losartan compound preparation of the present invention
[0033] Weighing lercanidipine hydrochloride bulk drug and losartan bulk drug and sieving for standby; lercanidipine hydrochloride is 0.5-40%, and losartan potassium is 6.25-50%.
[0034] Weigh lactose-hydrate, microcrystalline cellulose, hydroxyethyl starch sodium (type A), povidone K30, magnesium stearate, pregelatinized starch, colloidal silicon dioxide and stir to mix evenly; 20-40%, microcrystalline cellulose 10-40%, hydroxyethyl starch sodium (type A) 10-20%, povidone K302-8%, magnesium stearate 0.5-2.5%, pregelatinized starch 5-15%, colloidal silicon dioxide 1-10%
[0035] Vacuum compression, milling, sieving and granulation; tableting to control tablet core hardness.
[0036] Preparation of isolation coat solution: Take Opadry, add it to ethanol under stirring condition, stir until dispersed, then add purified water and stir to make isolation coat solution.
[0...
Embodiment 3
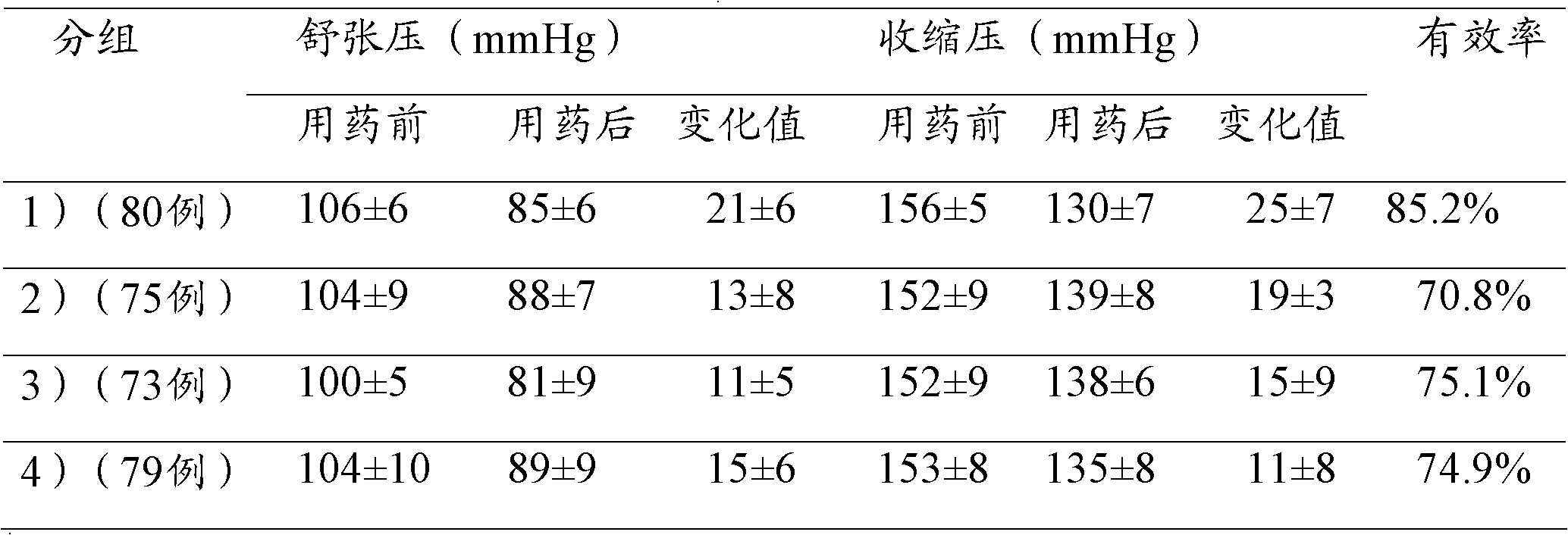
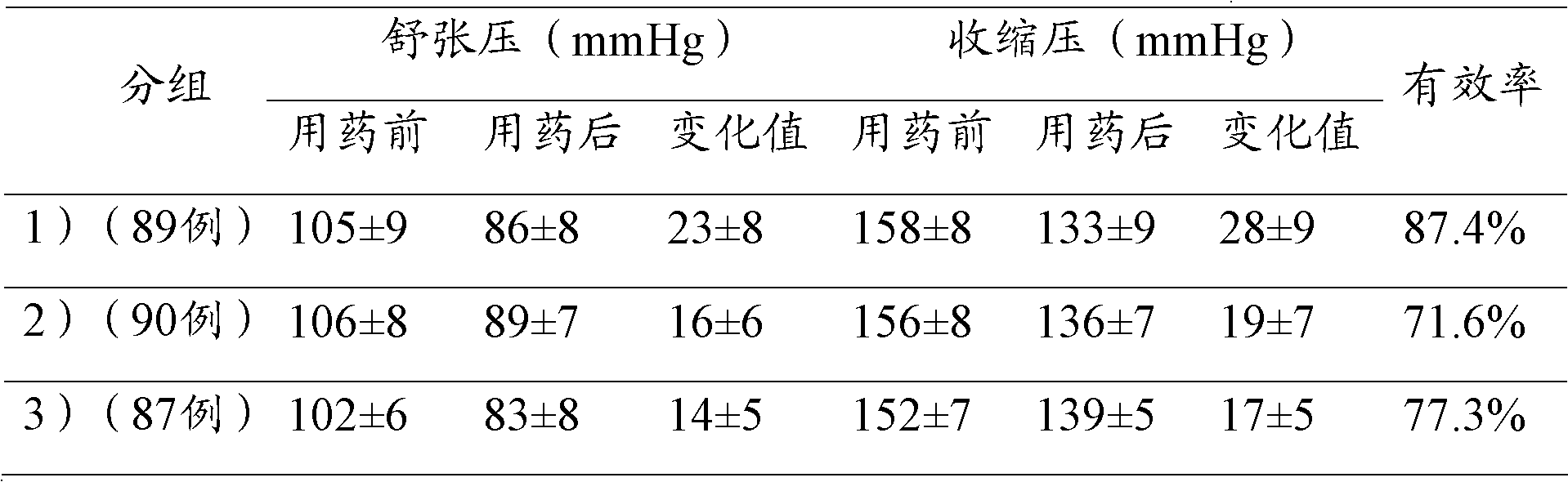
[0040] Embodiment 3: Carry out quality research to lercanidipine losartan compound preparation Wherein the content determination of lercanidipine hydrochloride,
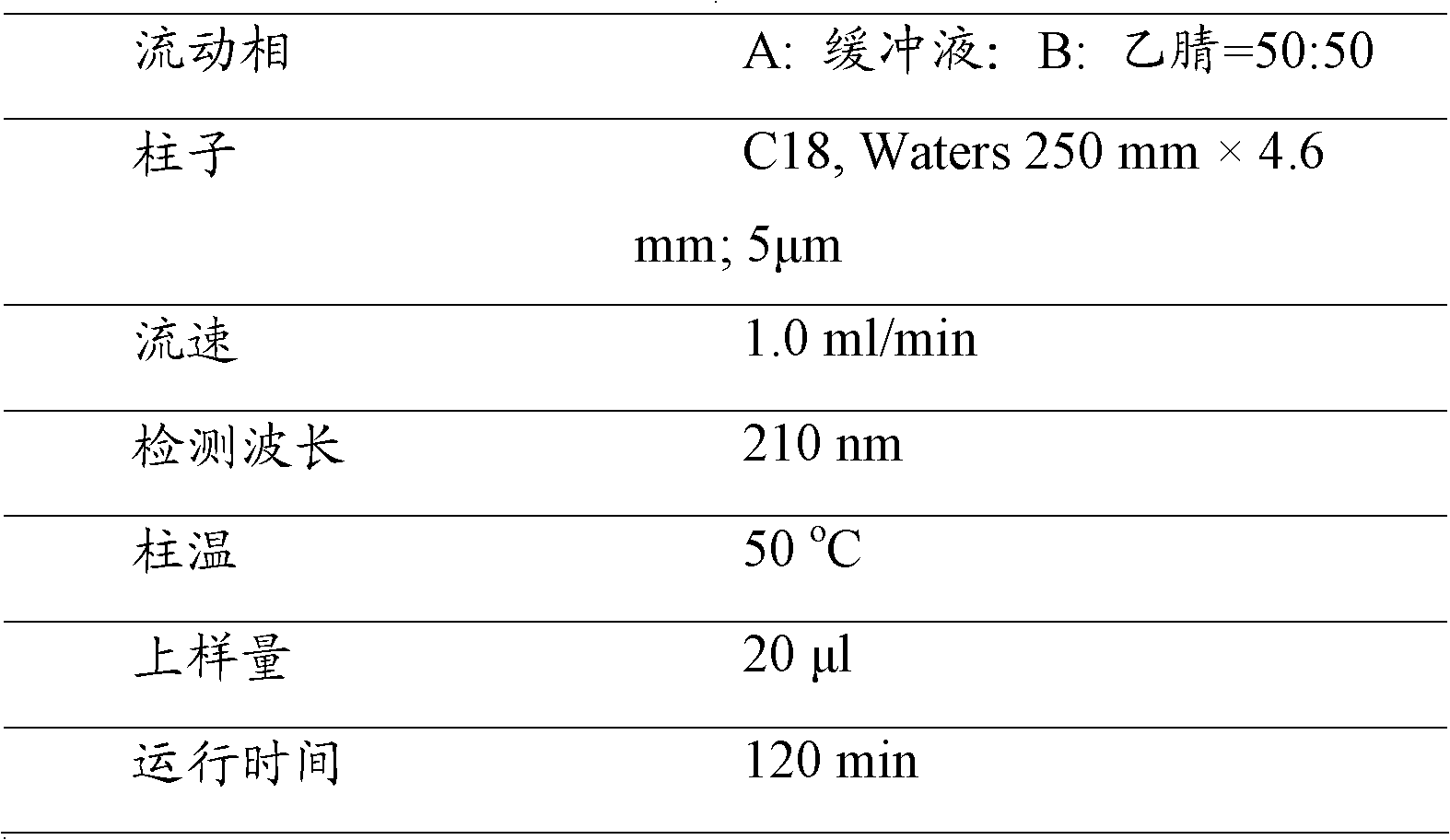
[0041] The HPLC method was used to determine the content of lercanidipine hydrochloride in the lercanidipine-losartan compound preparation prepared in Example 1. The liquid phase detection conditions are shown in Table 1.
[0042] The HPLC assay condition of lercanidipine hydrochloride in the compound preparation of table 1
[0043]
[0044]Preparation method of buffer A: dissolve 1.36g of potassium dihydrogen phosphate in 1L of water, add 1g of octane sulphonic acid sodium salt (octane sulphonic acid sodium salt) after dissolving, and use 10% phosphoric acid (v / v) for ultrasonic treatment Adjust the pH to 2.5 ± 0.05.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com