Preparation method of isopropyl 2-(3-nitrobenzal) acetoacetate
A technology of isopropyl acetoacetate and isopropyl acetate, which is applied in the field of preparation of isopropyl 2-acetoacetate, can solve the problems of a large amount of acid waste water, pollute the environment, and consume a large amount of concentrated acid, so as to reduce production costs and reduce Effects of Environmental Pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
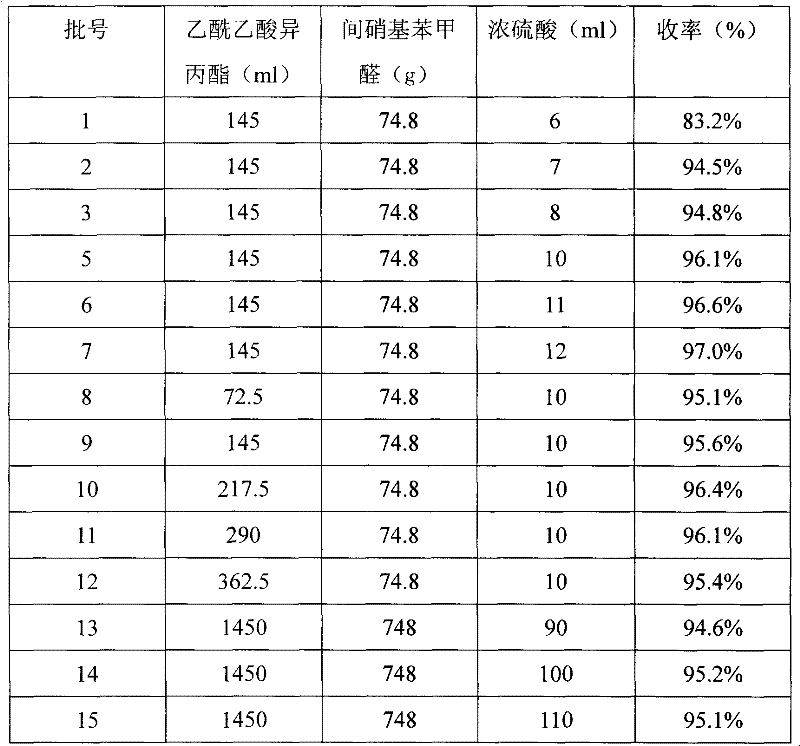
[0009] Take seven 500ml three-neck round-bottom flasks, add 145ml of isopropyl acetoacetate respectively, cool to 0°C, stir and add concentrated sulfuric acid 6, 7, 8, 9, 10, 11 and 12ml dropwise, and add in batches A total of 74.8g of nitrobenzaldehyde was stirred and reacted for 1 hour at 5°C. 0 degrees for 12 hours. Suction filtration, washing with purified water to neutrality, drying and separation of crude products. Dissolve the crude product in 100ml of isopropanol, stir and heat to 50°C, and keep it warm for 5 hours. Cool down, crystallize for 6 hours, filter with suction, and dry to obtain white solids 1-7, respectively.
Embodiment 2
[0011] Take four 500ml three-neck round bottom flasks, add 72.5, 145, 217.5, 290, 362.5ml of isopropyl acetoacetate respectively, cool to 4 degrees, stir and add 10ml of concentrated sulfuric acid dropwise, add m-nitrobenzene in batches A total of 74.8g of formaldehyde was stirred and reacted at 8 degrees for 12 hours. Place it at 10 degrees for 72 hours. Suction filtration, washing with alkaline aqueous solution until neutral, and drying to obtain the crude product. Dissolve the crude product in 200ml of isopropanol, stir and heat to 89°C, and keep it warm for 1 hour. Cool down, crystallize for 24 hours, filter with suction, and dry to obtain white solid 8-11.
Embodiment 3
[0013] Take three 5-liter three-necked flasks, add 1450ml of isopropyl acetoacetate, cool to 2 degrees, stir and add 90, 100, 110ml of concentrated sulfuric acid dropwise, add 748g of m-nitrobenzaldehyde in batches, and stir at 7 degrees for 1 hour . 0 degrees for 48 hours. Suction filtration, washing with purified water to neutrality, drying and separation of crude products. Dissolve the crude product in 1500ml of isopropanol, stir and heat to 80°C, and keep it warm for 3 hours. Cool down, crystallize for 18 hours, filter with suction, and dry to obtain white solids 12-15, respectively.
[0014] The productive rates of Examples 1, 2 and 3 are shown in Table 1.
[0015] Table 1
[0016]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com