Preparation method of 4-amino-6-(trichloroethenyl)-1, 3-benzene disulfonamide
A technology of trichlorovinyl and benzenedisulfonamide, which is applied in the preparation of 4-amino-6--(trichloroethenyl)-1,3-benzenedisulfonamide and the field of preparation of veterinary drugs, which can solve the problem of low synthesis efficiency , long cycle and other issues to achieve the effect of speeding up the process and productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
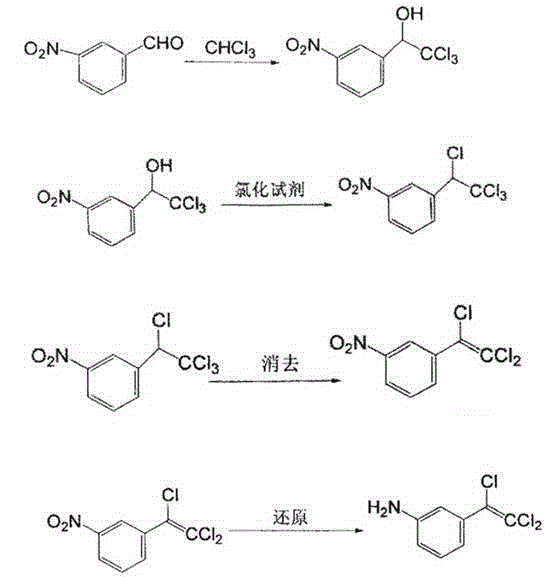
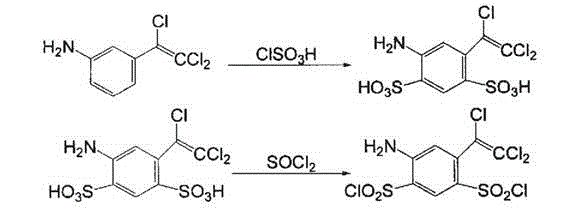
[0015] Put 30g of m-nitrobenzaldehyde and 15ml of chloroform in 120ml of DMF, cool to -10~-20°C, dissolve 0.9g of cesium carbonate in 20ml of methanol, add dropwise under nitrogen, and keep at -15~-20°C for 2 -3 hours transferred to 200mL toluene and 20mL30% hydrochloric acid solution, the water layer was separated, the organic layer was washed with water, and the water layer was separated; the organic layer was washed with sodium bicarbonate, purified, and then dried to obtain a condensate with a yield of 88 %; After the condensate is chlorinated and eliminated, 50g of the eliminated product is made of methanol-water as a solvent and iron is used as a reducing agent under acidic conditions, and 0.25g of iridium complex [Cp*IrCl 2 ] 2 As a catalyst, a dark red liquid is obtained through reduction reaction at room temperature, with a yield of 92%. Finally, the reduced product is sulfonated with chlorosulfonic acid, ammonified, and purified to obtain 4-amino-6--(trichloroethenyl...
Embodiment 2
[0017] Put 30g of m-nitrobenzaldehyde and 15ml of chloroform in 120ml of DMF, cool to -10~-20°C, dissolve 1.5g of cesium carbonate in 20ml of methanol, add dropwise under nitrogen, and keep at -15~-20°C for 2 -3 hours transferred to 200mL toluene and 20mL30% hydrochloric acid solution, the water layer was separated, the organic layer was washed with water, and the water layer was separated; the organic layer was washed with sodium bicarbonate, purified, and then dried to obtain a condensate with a yield of 90 %; After the condensate is chlorinated and eliminated, 50g of the eliminated product is made of methanol-water as a solvent and iron is used as a reducing agent under acidic conditions, and 0.5g of iridium complex [Cp*IrCl 2 ] 2 As a catalyst, a dark red liquid can be obtained through reduction reaction at room temperature, with a yield of 96%. Finally, the reduced product can be sulfonated with chlorosulfonic acid, ammonified, and purified to obtain 4-amino-6--(trichloro...
Embodiment 3
[0019] Put 30g of m-nitrobenzaldehyde and 15ml of chloroform in 120ml of DMF, cool to -10~-20°C, dissolve 1.2g of cesium carbonate in 20ml of methanol, add dropwise under nitrogen, and keep at -15~-20°C for 2 -3 hours transferred to 200mL toluene and 20mL30% hydrochloric acid solution, the water layer was separated, the organic layer was washed with water, and the water layer was separated; the organic layer was washed with sodium bicarbonate, purified, and then dried to obtain a condensate with a yield of 89% %; After the condensate is chlorinated and eliminated, 50g of the eliminated product is made of methanol-water as a solvent and iron is used as a reducing agent under acidic conditions, and 0.4g of iridium complex [Cp*IrCl 2 ] 2 As a catalyst, a deep red liquid can be obtained through reduction reaction at room temperature, with a yield of 90%. Finally, the reduced product can be sulfonated with chlorosulfonic acid, ammonified, and purified to obtain 4-amino-6--(trichlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com