Technique of preparing m-nitrobenzene acetylene
A technology for the preparation of nitrophenylacetylene, which is applied in the field of preparation of m-nitrophenylacetylene, can solve the problems of long-time heating, long reaction time, instability of m-nitroiodobenzene, etc., and achieves mild reaction conditions and short reaction time. Short, easy post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
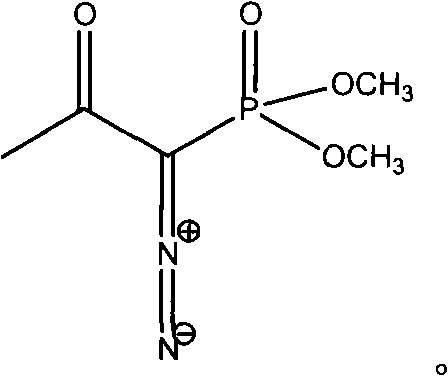
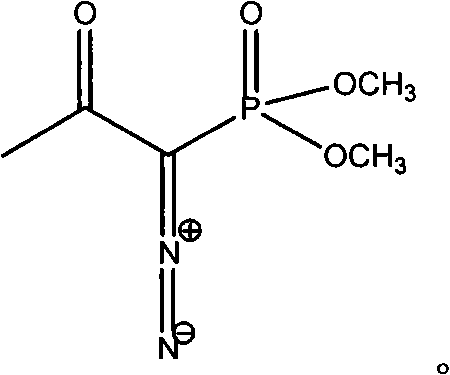
[0017] Dissolve 6 g of m-nitrobenzaldehyde in 80 ml of anhydrous methanol, blow nitrogen, add 11 g of anhydrous potassium carbonate and 10.6 g of Bestmann-Ohira reagent, stir and react at room temperature for 24 hours under nitrogen atmosphere, filter, collect the filtrate, and evaporate under reduced pressure Organic solvent, the residue was dissolved in 20ml of dichloromethane, the dichloromethane solution was washed successively with 240ml of 5% sodium bicarbonate and saturated brine, the organic layer was separated, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain a light yellow Nitrophenylacetylene solid 5.5g, mp25-26°C, yield 93.5%.
Embodiment 2
[0019] Dissolve 6 g of m-nitrobenzaldehyde in 100 ml of anhydrous methanol, pass through nitrogen, add 12.8 g of anhydrous potassium carbonate and 12.5 g of Bestmann-Ohira reagent, stir and react at room temperature for 24 h under nitrogen atmosphere, filter, collect the filtrate, and evaporate under reduced pressure Remove the organic solvent, dissolve the residue with 30ml of ethyl acetate, wash the ethyl acetate solution with 240ml of 5% sodium bicarbonate and saturated brine successively, separate the organic layer, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure to obtain light yellow m-nitrophenylacetylene solid 5.6g, mp25-26.5°C, yield 95.2%.
Embodiment 3
[0021] Dissolve 5 g of m-nitrobenzaldehyde in 80 ml of anhydrous methanol, pass through nitrogen, add 10 g of anhydrous potassium carbonate and 8 g of Bestmann-Ohira reagent, stir and react at room temperature under nitrogen atmosphere for 24 h, filter, collect the filtrate, and evaporate organic matter under reduced pressure. Solvent, the residue was dissolved in 30ml of chloroform, the chloroform solution was washed successively with 200ml of 5% sodium bicarbonate and saturated brine, the organic layer was separated, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain a light yellow m-nitrophenylacetylene solid 4.3g, mp25-26.5°C, yield 88.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com