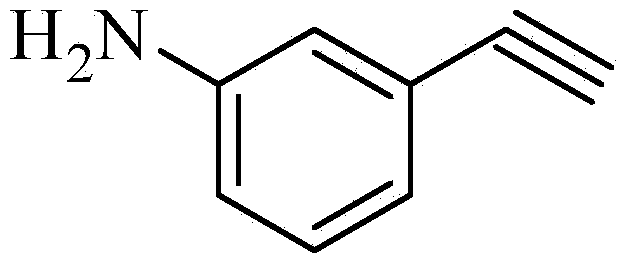
Preparation method for m-aminophenylacetylene
A technology of aminophenylacetylene and nitrophenylacetylene, which is applied in the field of preparation of m-aminophenylacetylene, can solve the problems of unsuitability for industrial production, difficult control of reaction conditions, high product cost, etc., achieve simple and efficient reaction process, reduce production cost, The effect of little environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
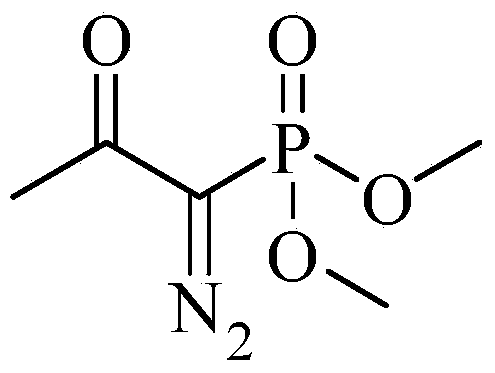
[0034]Preparation of dimethyl (1-diazo-2-oxopropyl)phosphonate (Bestmann-Ohira reagent). Add 33.2g (0.2mol) of dimethyl acetonyl phosphate and 300ml toluene in sequence in a 1000ml reaction flask, add 5.1g (0.21mol) of NaH in batches, and after all the gas is released, p-toluenesulfonyl azide A mixture of 41.4g (0.21mol) and 500ml of tetrahydrofuran was added to the reaction solution and reacted at room temperature for 16h. After the reaction, dilute with petroleum ether, filter, wash the filter cake with diethyl ether, evaporate the solvent under reduced pressure to obtain 33.1 g of yellow liquid with a yield of 86.5%.
Embodiment 2
[0036] Preparation of dimethyl (1-diazo-2-oxopropyl)phosphonate (Bestmann-Ohira reagent). Add 33.2 g (0.2 mol) of dimethyl acetonyl phosphate and 300 ml tetrahydrofuran in sequence in a 1000 ml reaction bottle, add 8 g (0.2 mol) of sodium hydride in batches, and after all the gas is released, p-toluenesulfonyl azide A mixture of 47.3g (0.24mol) and 500ml tetrahydrofuran was added to the reaction solution and reacted at room temperature for 12h. After the reaction, add petroleum ether to dilute, filter, wash the filter cake with ether, evaporate the solvent under reduced pressure, and obtain 33.7 g of yellow liquid with a yield of 88%.
Embodiment 3
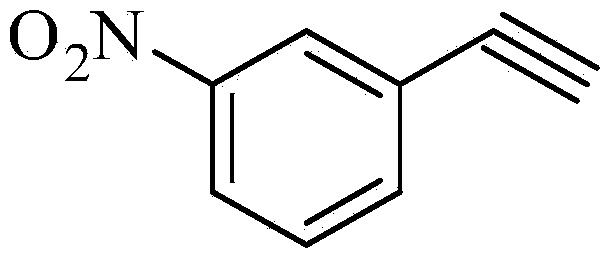
[0038] Preparation of m-nitrophenylacetylene. Add 15.1 g (0.1 mol) of m-nitrobenzaldehyde and 18.3 g of homemade (1-diazo-2-oxopropyl)phosphonic acid dimethyl ester (Bestmann-Ohira reagent) in a dry and clean 1000 ml reaction bottle (0.11mol) (purified) and 750ml of methanol, cooled to 0-5°C, slowly added 20.7g (0.15mol) of anhydrous potassium carbonate, the temperature was controlled at 0-5°C during addition, after the addition, keep stirring for 2h. Then the reaction temperature was raised to room temperature, and the stirring reaction was continued for 8h. Stop the reaction, add saturated ammonium chloride aqueous solution, dichloromethane 150ml×3 extract, combine the organic phases, and dry over anhydrous sodium sulfate for 2h. Filter, wash the filter cake with an appropriate amount of dichloromethane, combine the organic phases, concentrate under reduced pressure to recover the solvent, freeze and solidify to obtain 13.1 g of a light yellow solid, with a yield of 89.2%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com