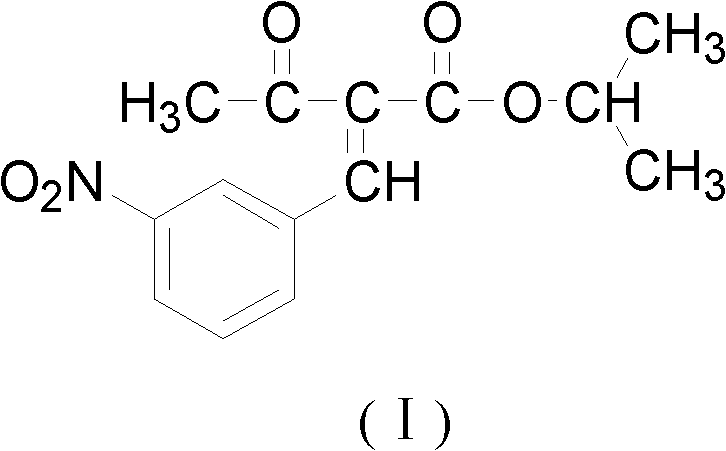
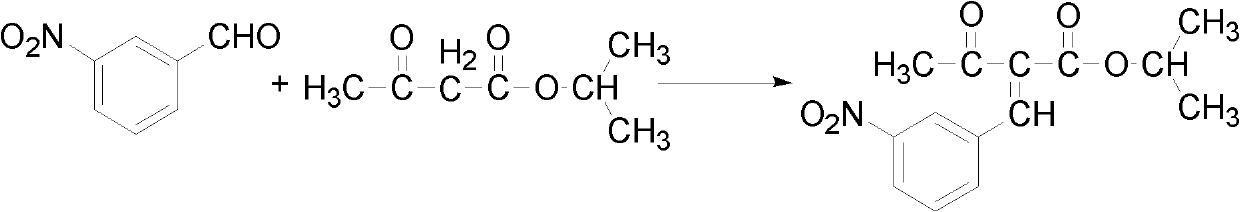
Preparation method of isopropyl 2-(3-nitrophenylmethylene)acetacetate
A technology of isopropyl acetoacetate and nitrobenzylidene is applied in the field of preparation of pharmaceutical intermediate compounds, can solve the problems of low yield and high reaction temperature, achieve high yield, mild reaction conditions, and reduce production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add m-nitrobenzaldehyde (151g, 1.0mol) and isopropyl acetoacetate (144g, 1.0mol) in a three-necked flask equipped with mechanical stirring, reflux condenser, and thermometer, and dissolve in 500ml isopropanol, add 14.5g Catalytic amount of piperidinium acetate, stirred at -20~-10°C for 24h, and left overnight. After filtration, 21.61g of the finished product was obtained, with a yield of 78%.
Embodiment 2
[0042] Add m-nitrobenzaldehyde (151g, 1.0mol) and isopropyl acetoacetate (144g, 1.0mol) in a three-necked flask equipped with mechanical stirring, reflux condenser, and thermometer, and dissolve in 500ml isopropanol, add 14.5g Catalytic amount of piperidinium acetate, stirred at -10~5°C for 4 hours, and left overnight. After filtration, 23.27g of the finished product was obtained, with a yield of 84%.
Embodiment 3
[0044] Add m-nitrobenzaldehyde (151g, 1.0mol) and isopropyl acetoacetate (144g, 1.0mol) in a three-necked flask equipped with mechanical stirring, reflux condenser, and thermometer, and dissolve in 500ml isopropanol, add 14.5g Catalytic amount of piperidinium acetate, stirred at 5-45°C for 1h, and left overnight. After filtration, 22.16g of the finished product was obtained, with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com