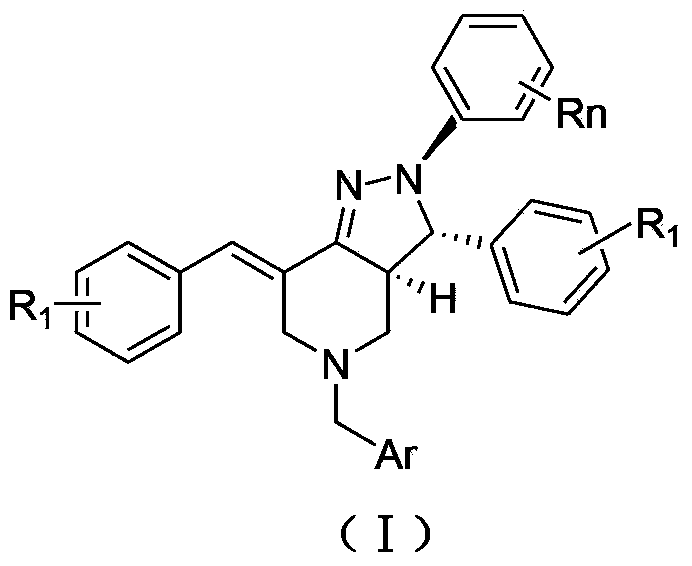
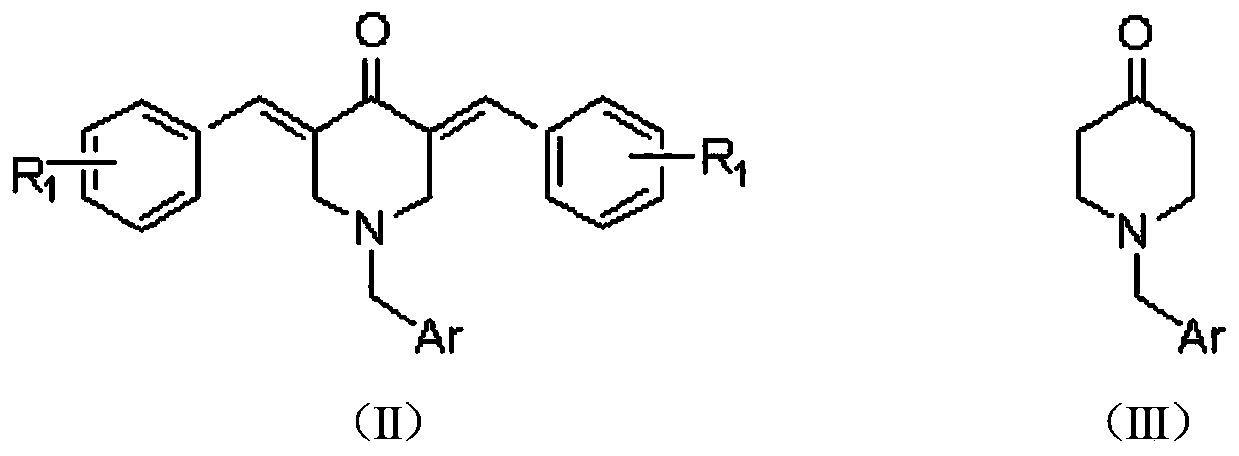
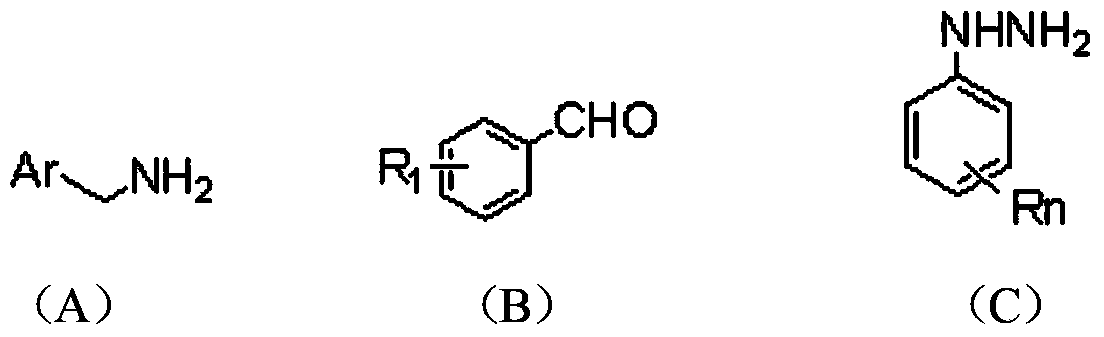
2, 3, 5, 7-tetrasubstituted dihydro-pyrazolo piperidine derivative and preparation method and application thereof
A technology of dihydropyrazole and hexahydropyridine, which is used in drug combination, organic chemistry, antitumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] 2. The preparation method of the embodiment of the general formula (I) of the present invention
[0040] (1) At room temperature, add 0.16mol methyl acrylate and 7mL methanol to a 100mL three-neck bottle, and slowly add the mixture of 0.04mol substituted aromatic methylamine represented by formula (A) and 4mL methanol into the three-neck bottle while stirring During the process, the temperature of the reaction system should not exceed 50°C. After the dropwise addition, heat and reflux for 8 hours. After the reaction is over, recover methanol and unreacted methyl acrylate, distill under reduced pressure, and obtain light yellow oily liquid N,N-bis(β-methyl acrylate ) Aromatic methylamine.
[0041]Add 15mL of anhydrous toluene and 0.122mol of sodium metal to a 250mL dry three-necked flask, stir and heat to reflux, add 0.2mL of anhydrous methanol, and then slowly add 0.04mol of N,N-bis(β-methyl propionate) aromatic A mixture of methylamine and 20 mL of anhydrous toluene....
Embodiment 1
[0046] Example 1: 2-(4-nitrophenyl)-3-(4-fluorophenyl)-5-(2-furylmethyl)-7-(4-fluorobenzylidene)-2H-pyrazole And [4,3-c] hexahydropyridine (Ⅰ 1 )
[0047] Yield 86%; melting point 245-246°C; 1 H NMR (CDCl 3 ,400MHz)δ2.43-2.49(m,1H),3.16-3.42(m,3H),3.43-3.63(m,2H),4.06(d,J=14.1Hz,1H),4.47(d,J= 12.7Hz,1H),6.11(s,1H),,6.37(s,1H),6.82(t,J=7.2Hz,1H),7.01-7.74(m,13H).IR(KBr,cm -1 )3445,1640,1597,1495,1436,1082,747;;Anal.Calcd for C 30 h 24 f 2 N 4 o 3 :C,68.43;H,4.59;N,10.64.Found:C,68.45;H,4.63;N,10.61.
Embodiment 2
[0048] Example 2: 2-(3-nitrophenyl)-3-(4-fluorophenyl)-5-(2-furylmethyl)-7-(4-fluorobenzylidene)-2H-pyrazole And [4,3-c] hexahydropyridine (Ⅰ 2 )
[0049] Yield 77%; melting point 259-260°C; 1 HNMR(400MHz,)δ2.44-2.51(m,1H),3.15-3.33(m,3H),3.47-3.63(m,2H),4.11(d,J=14.3Hz,1H),4.42(d, J=12.4Hz,1H),6.13(s,1H),,6.37(s,1H),6.87(t,J=7.2Hz,1H),7.03-7.72(m,13H);IR(KBr,cm -1 )3447,1641,1598,1495,1436,1081,748;Anal.Calcd for C 30 h 24 f 2 N 4 o 3 :C,68.43;H,4.59;N,10.64;Found:C,68.41;H,4.61;N,10.61.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com