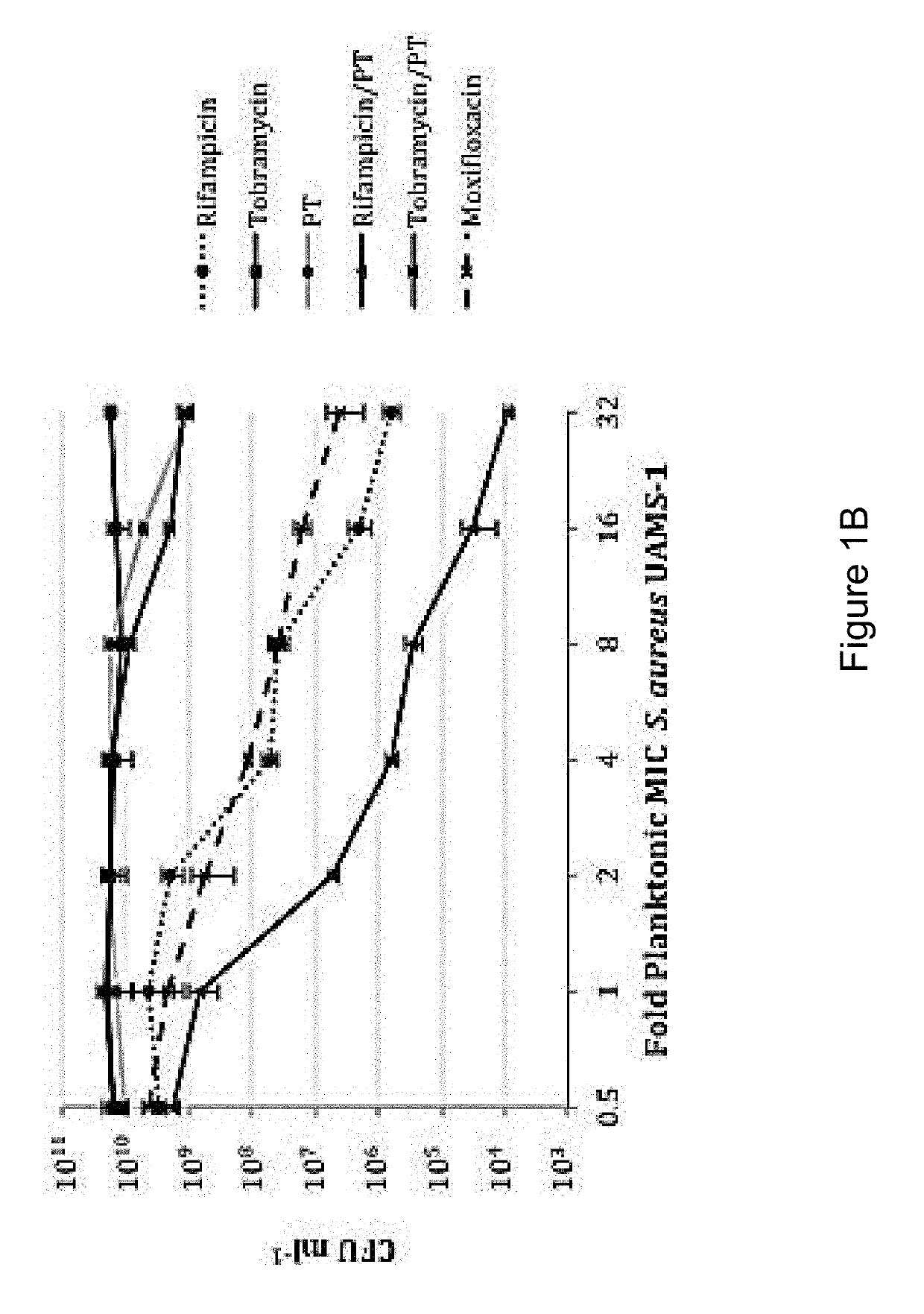
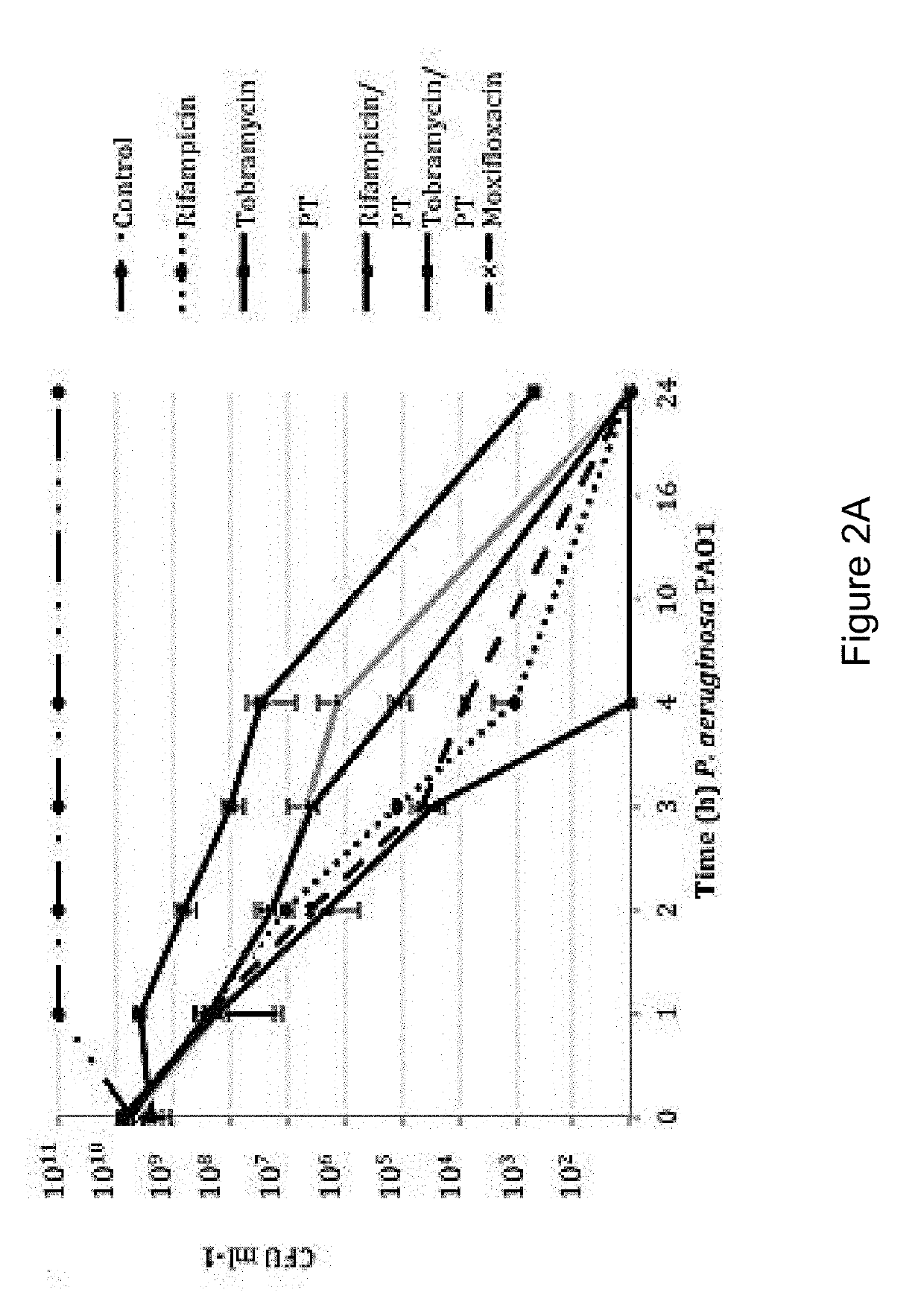
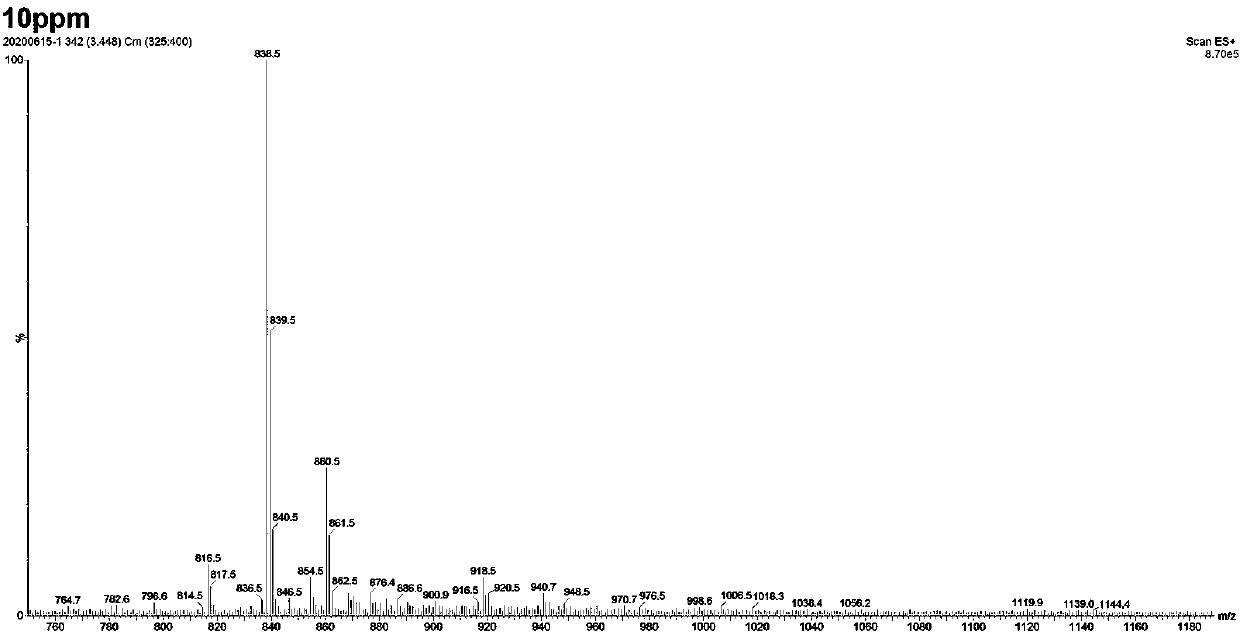
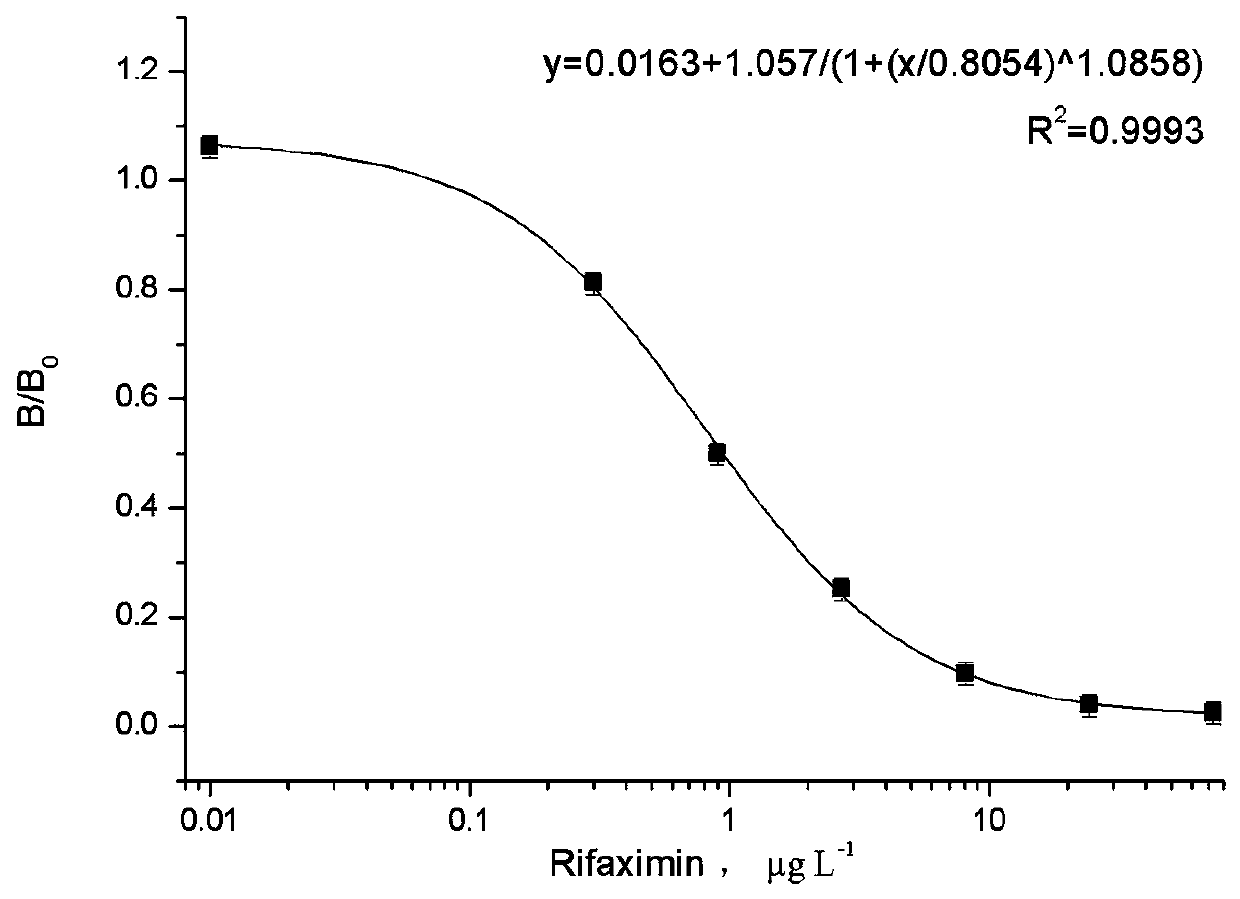
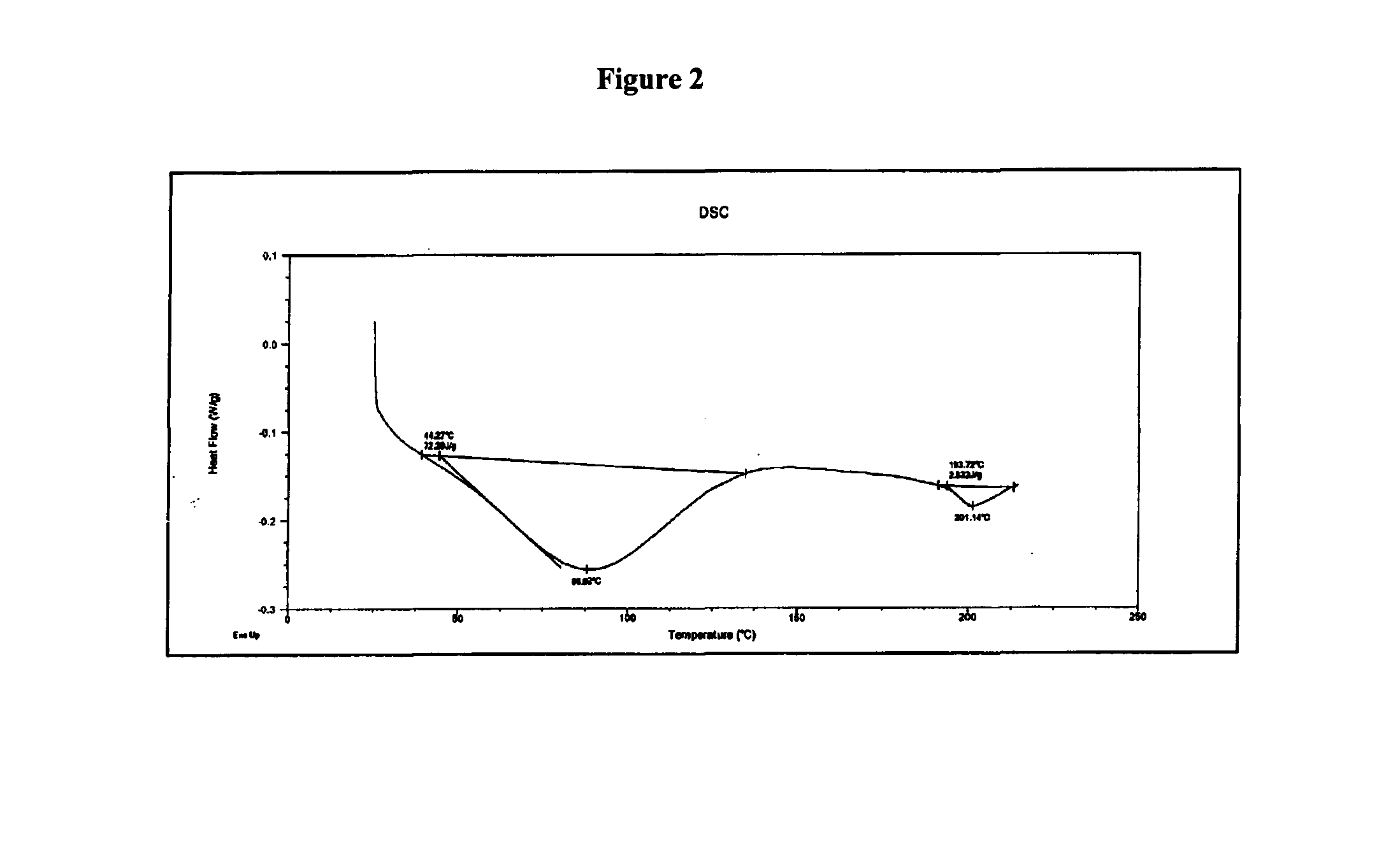
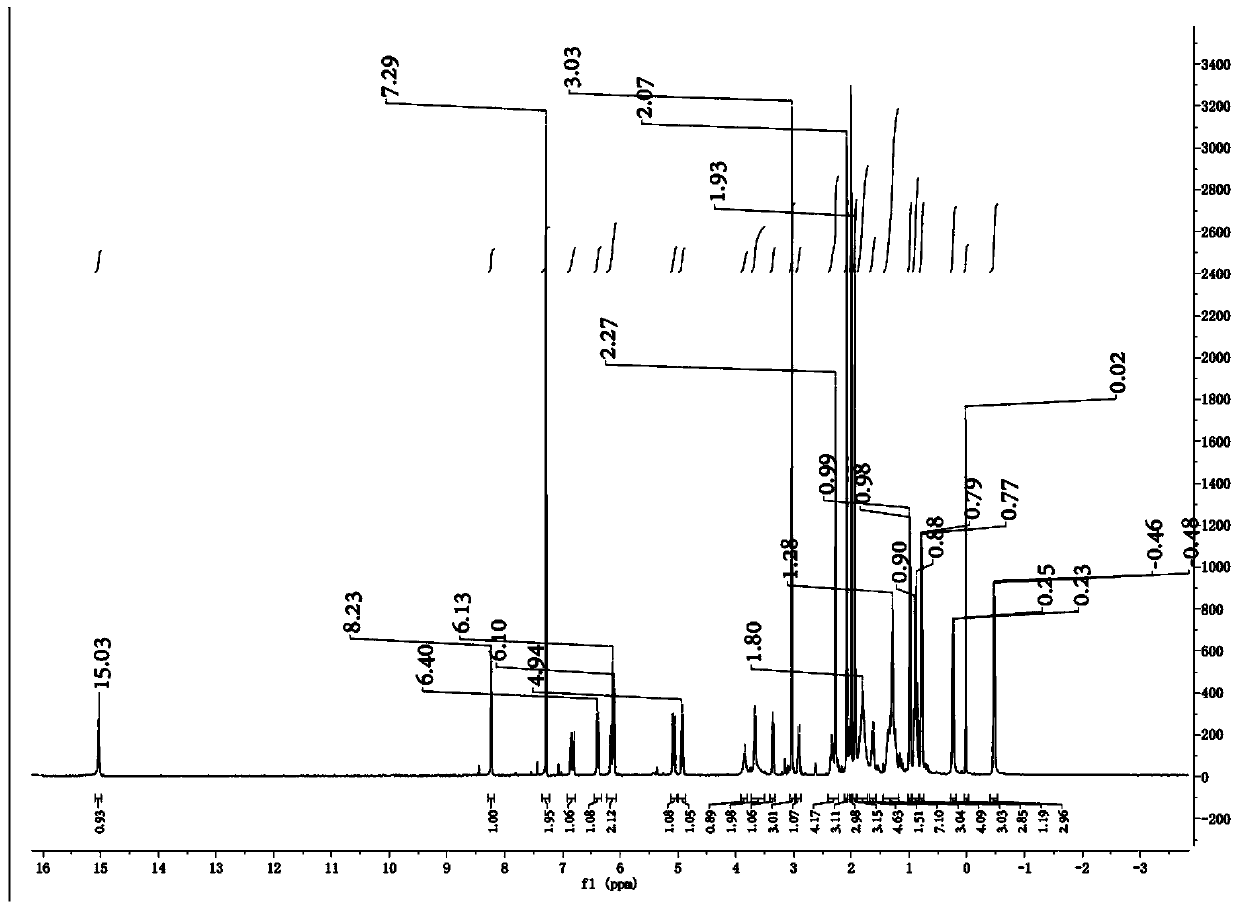
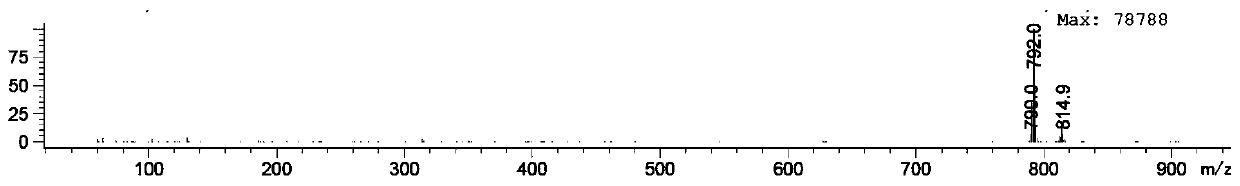
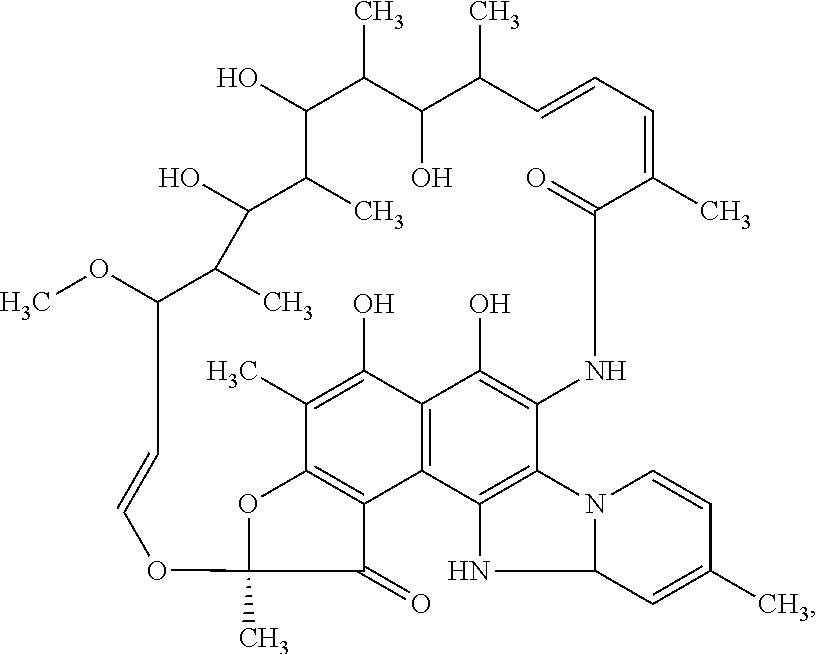
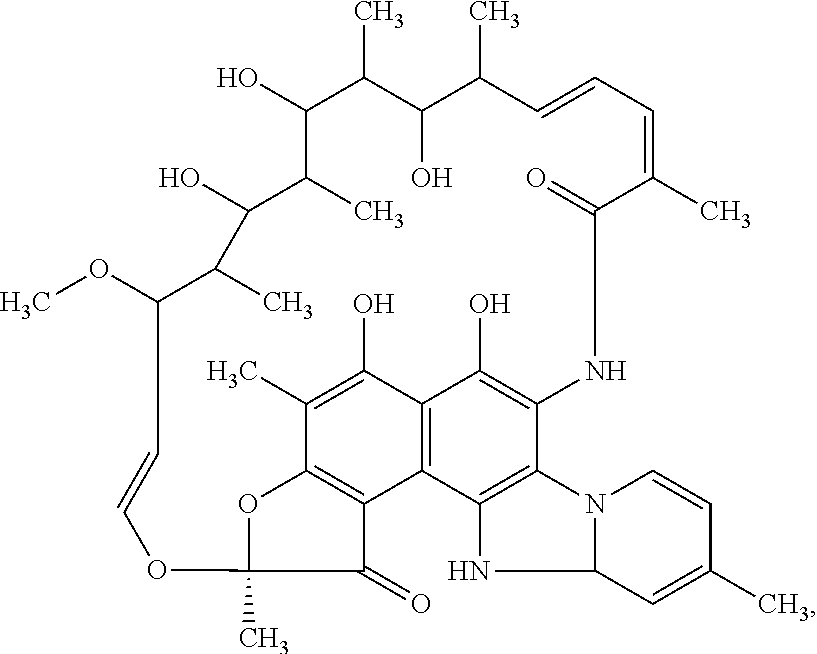
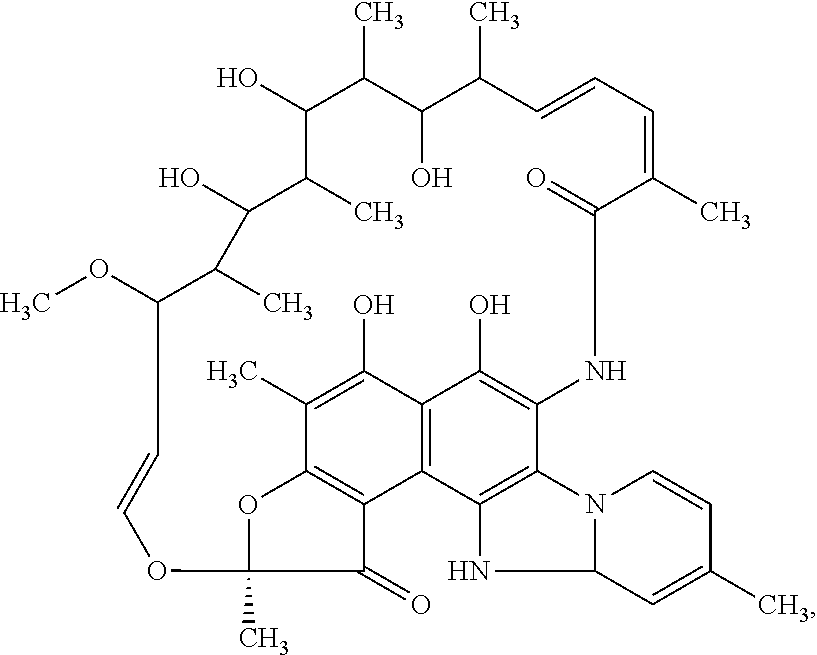
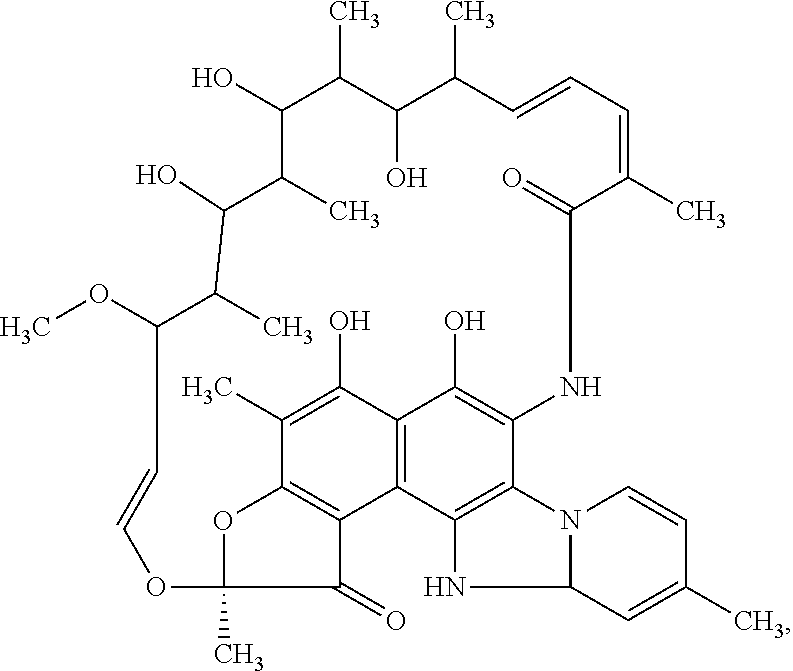
Patents
Literature
45 results about "Rifaximine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmaceutical compositions of rifaximin
ActiveUS20090028940A1Extended stayImprove complianceAntibacterial agentsBiocideImmediate releasePharmaceutical medicine
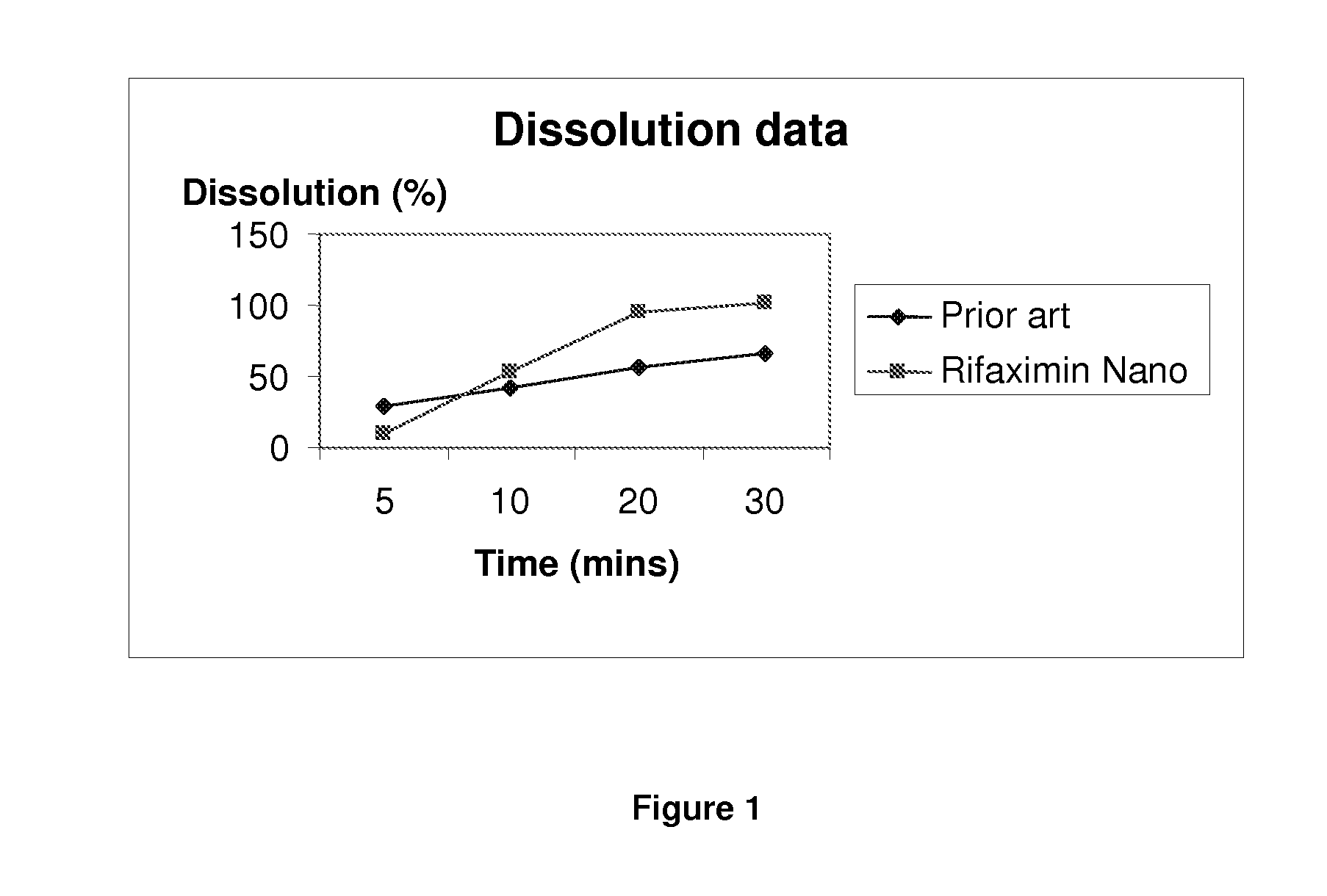
A pharmaceutical composition comprising therapeutically effective amount of rifaximin or pharmaceutically acceptable salt or enantiomer or polymorph thereof, pharmaceutically acceptable excipient(s) and release controlling agent(s). Pharmaceutical composition of rifaximin comprising: at least two entities wherein one entity is an immediate release or fast release and the other is controlled release. The pharmaceutical composition in the form of multilayer tablet comprising, at least one layer comprising, therapeutically effective amount of rifaximin or pharmaceutically acceptable salt or enantiomer or polymorph thereof, pharmaceutically acceptable excipient(s); said layer providing controlled release rifaximin; and at least one layer which provides increased residence time of the dosage form in the gastrointestinal tract. The pharmaceutical formulation comprising rifaximin having an in vitro dissolution profile, wherein about 70% of rifaximin is released in about 24 hours. The composition comprising therapeutically effective amount of rifaximin or pharmaceutically acceptable salt(s) or enantiomer(s) or polymorph(s) thereof, one or more release controlling agent(s) and pharmaceutically acceptable excipient(s) causing pathogenic eradication.
Owner:LUPIN LTD
Use of polyols to obtain stable polymorphous forms of rifaximin
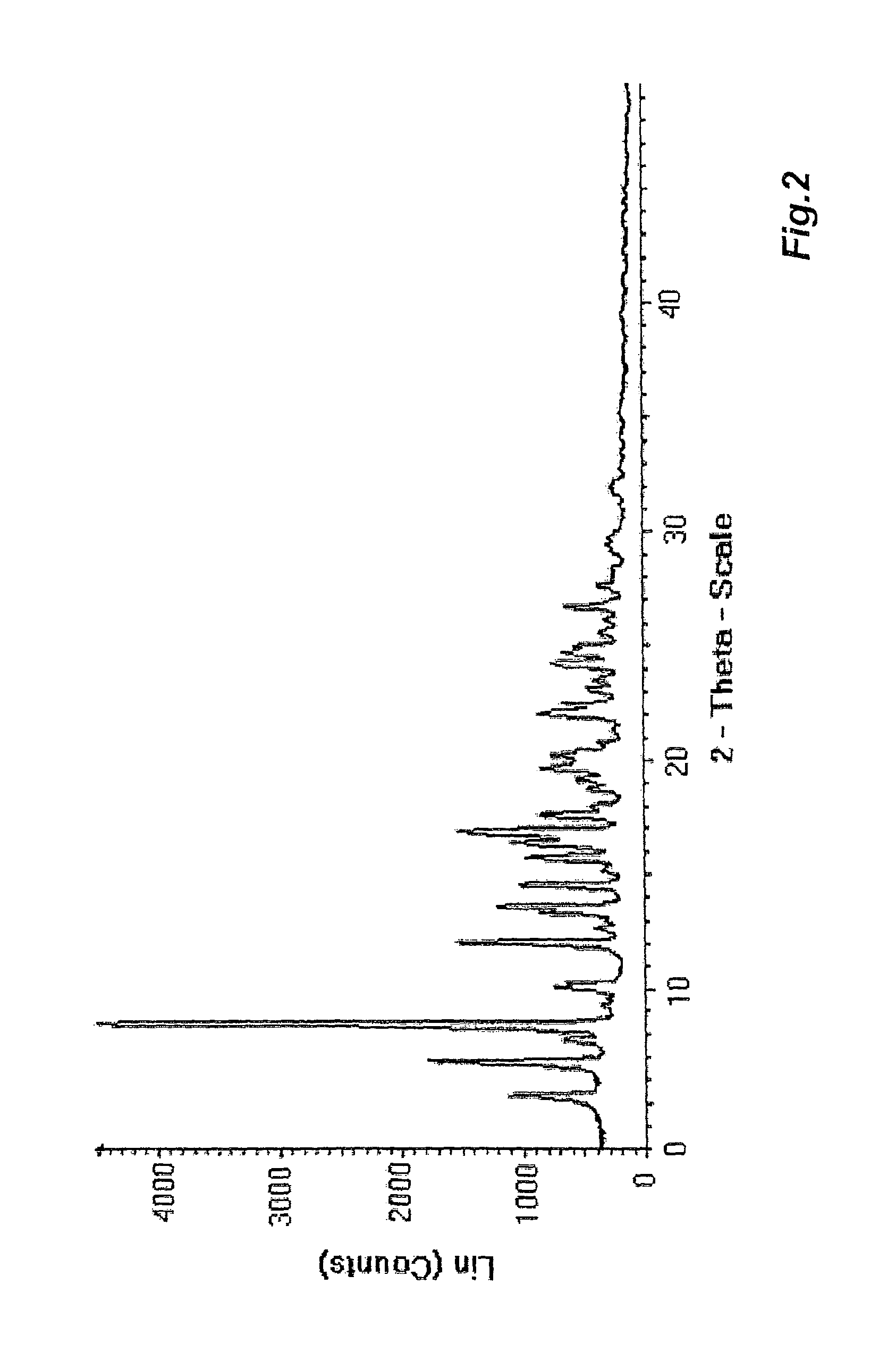
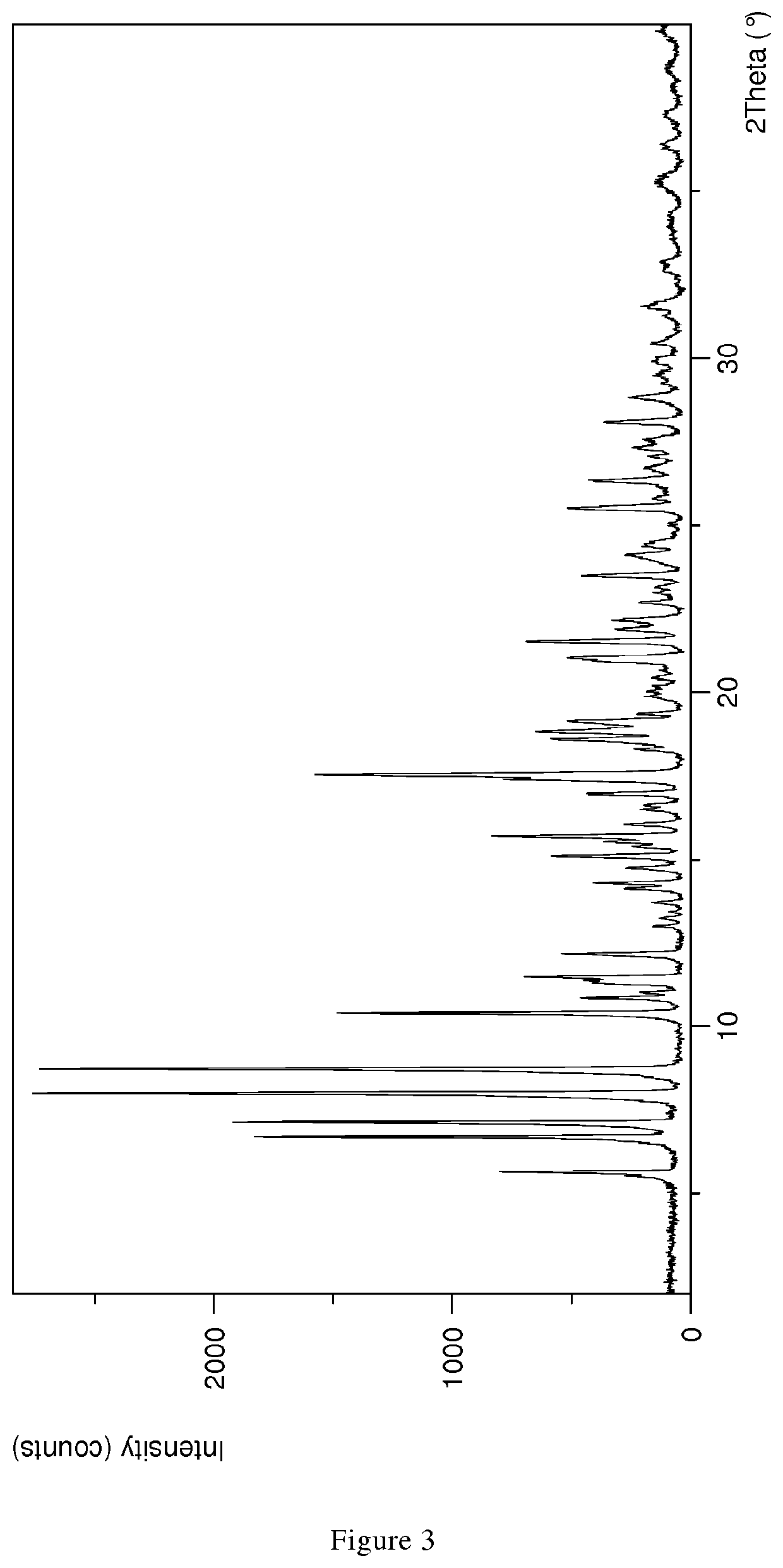
Polyols stabilize polymorphous form of rifaximin, in particular the β form. When polyols having at least two hydroxy groups are added to rifaximin powder, polymorph β is stable and remains stable in time independently from the environment humidity. In this invention a method to prepare formulations constituted by pure and stable polymorphous forms able to give a pharmaceutical product is described.
Owner:ALFASIGMA SPA
Method for the production of amorphous rifaximin
ActiveUS20120289532A1Large solubilityImprove drug bioavailabilityAntibacterial agentsBiocideHigh energyMedicine
The present invention relates to a new amorphous form of rifaximin and to methods for the preparation thereof by means of high energy milling or Spray drying. The present invention further relates to a new amorphous form for use as medicament and to the pharmaceutical compositions composing it.
Owner:UNILAB S A S DI LAVAGNA SILVIO MASSIMO & C
Agent for preventing and/or treating functional gastrointestinal disorder
ActiveUS8980872B2Improve and enhance motilityAbnormal functionBiocideOrganic chemistryFunctional disturbanceFunctional diarrhea
A method for preventing and / or treating a functional gastrointestinal disorder, comprising administering, to a subject with the functional gastrointestinal disorder, rifaximin as an effective ingredient. The functional gastroinstestinal disorder includes a functional esophageal disorder, a functional gastroduodenal disorder (e.g., a functional dyspepsia), a functional bowel disorder (e.g., a functional bloating, a functional diarrhea), a functional abdominal pain syndrome, a functional gallbladder and Sphincter of Oddi disorder, a functional anorectal disorder (e.g., a functional fecal incontinence, a functional anorectal pain, a functional defecation disorder), a functional disorder in neonates and toddlers (e.g., an infant functional diarrhea), a functional disorder in children and adolescents (e.g., a childhood functional abdominal pain, a childhood nonretentive fecal incontinence), and other diseases.
Owner:ALFASIGMA SPA
Polymorph of rifaximin and process for the preparation thereof
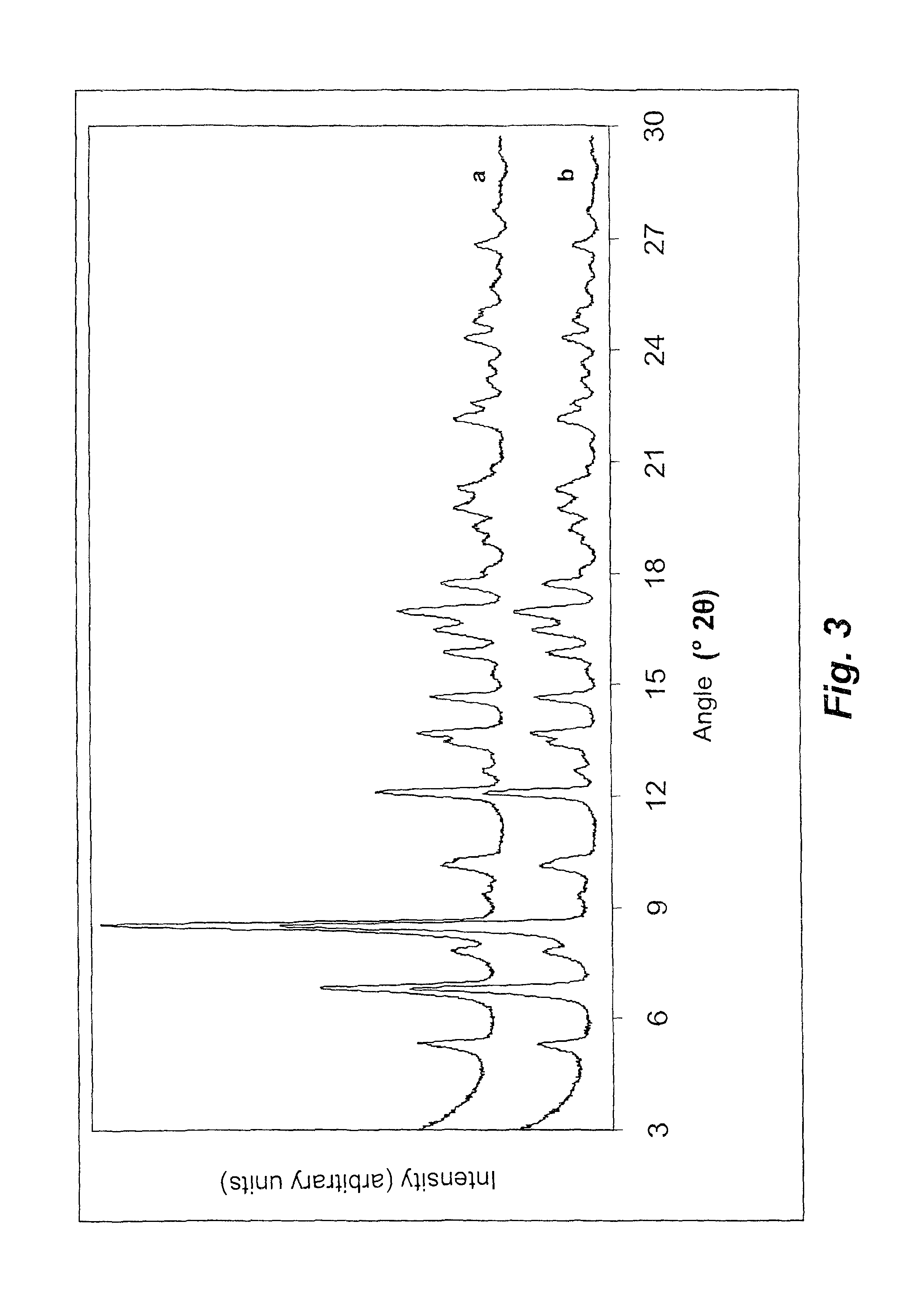
The present invention relates to a new polymorph of Rifaximin, designated κ, and to a process for the preparation thereof. Under certain aspects, the invention also relates to pharmaceutical compositions comprising an effective amount of the polymorphic form κ of Rifaximin and a pharmaceutically acceptable carrier and its uses in the treatment of gastrointestinal conditions.
Owner:CLAROCHEM IRELAND
New use of rifamycin-quinolizidone dual-target molecule
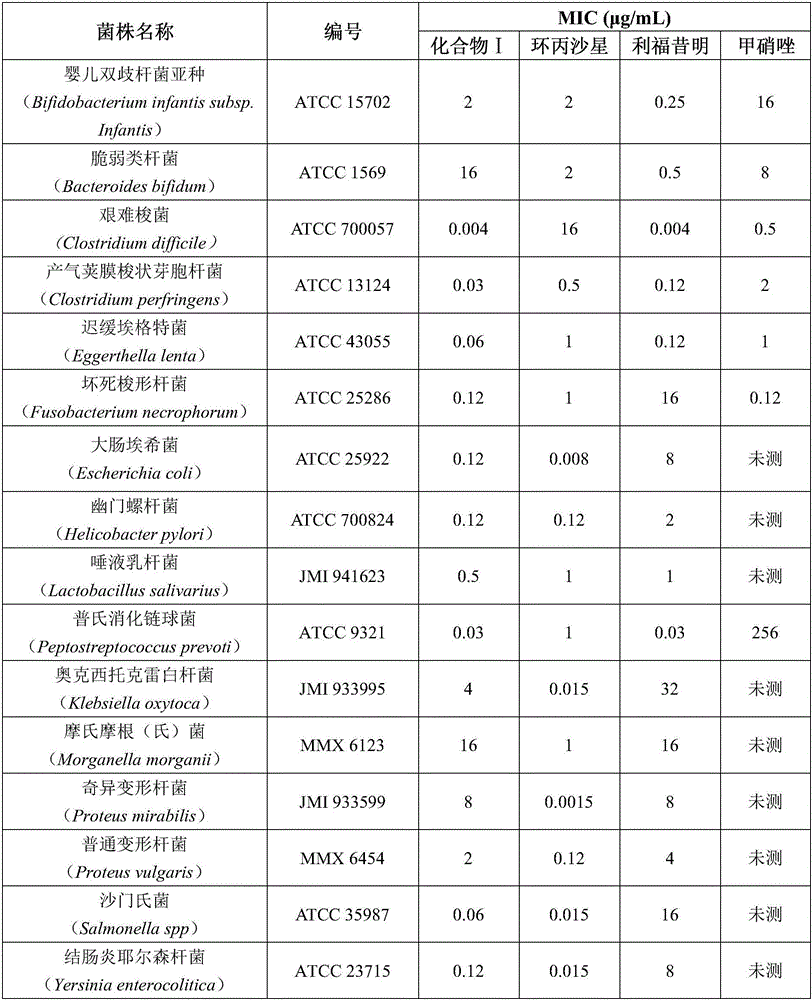
InactiveCN106822125AHigh antibacterial activityLow frequency of drug resistanceAntibacterial agentsOrganic active ingredientsAntibacterial activityHepatic encephalopathy
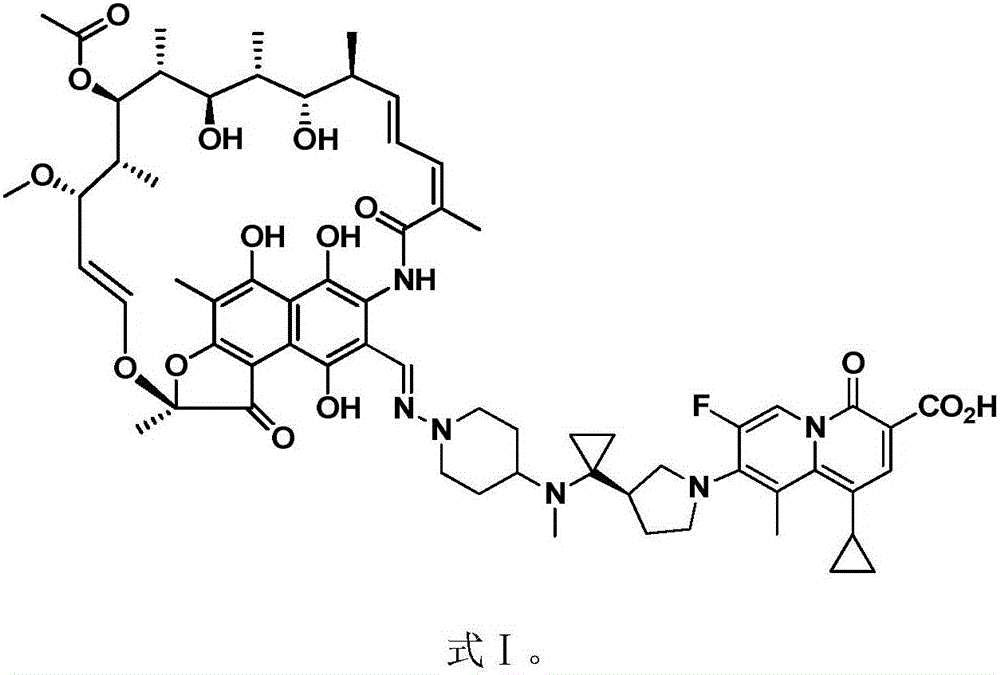
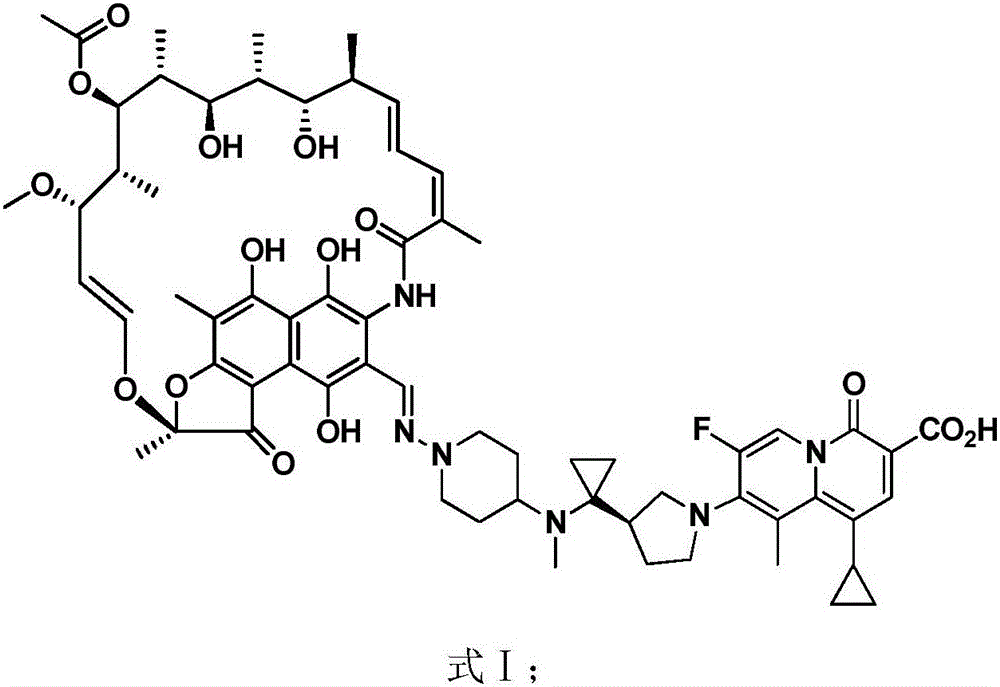
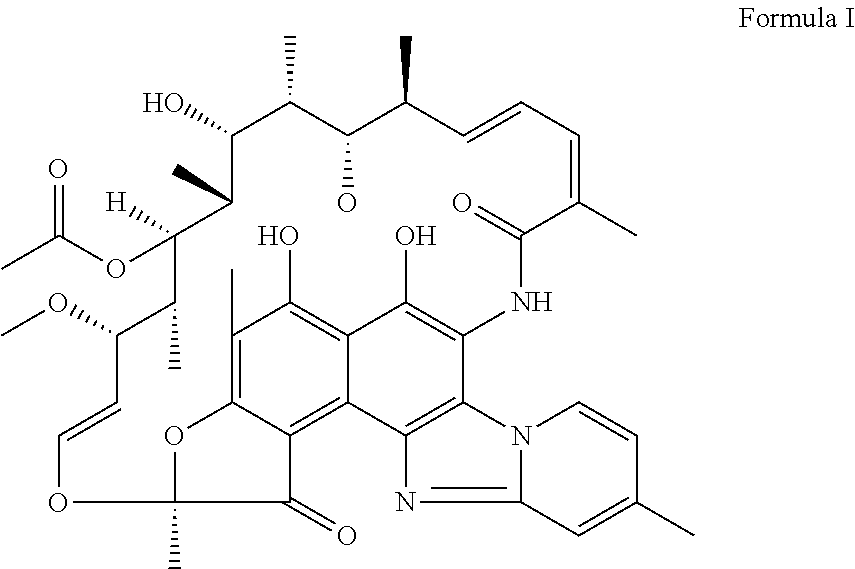
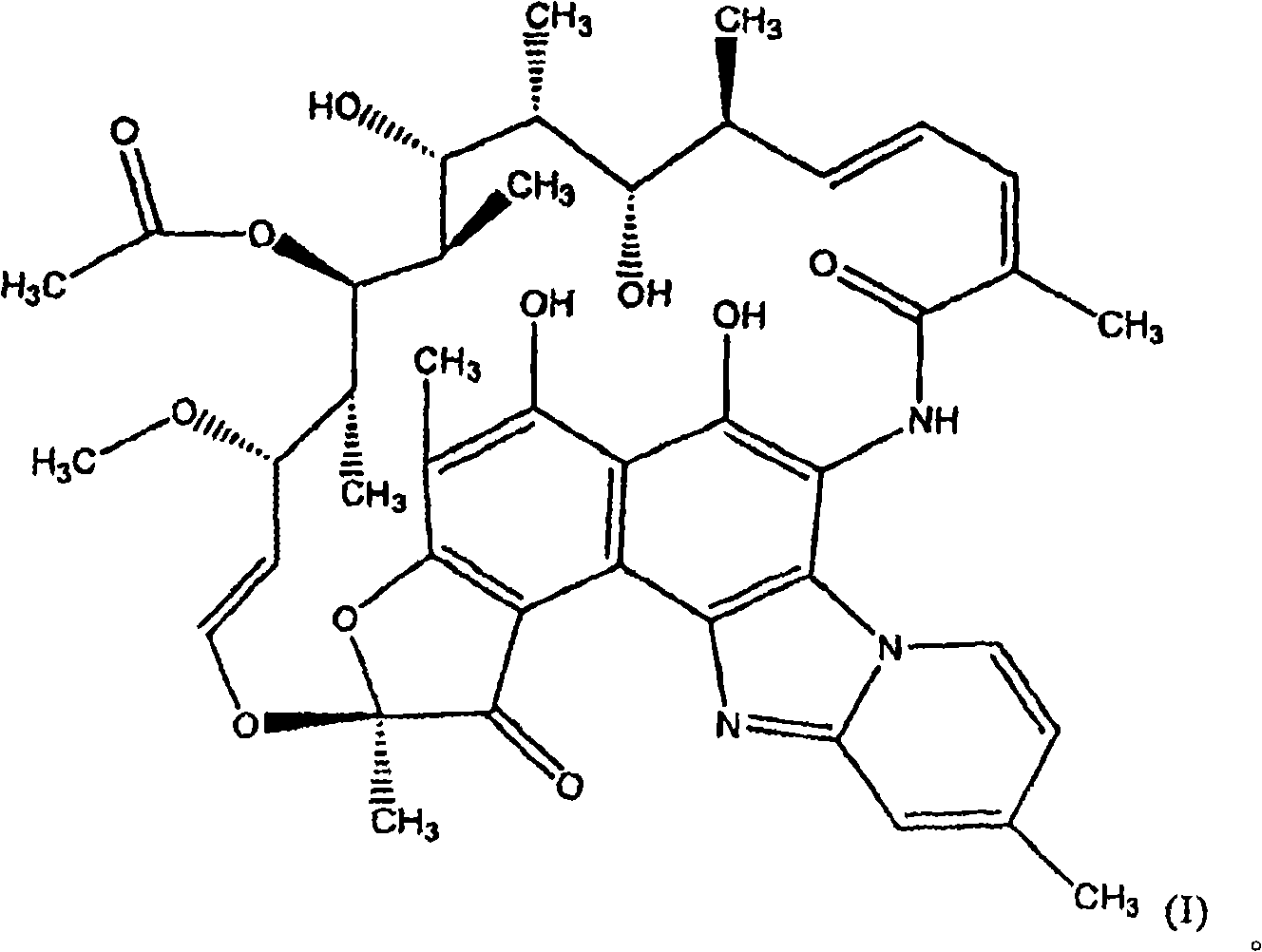
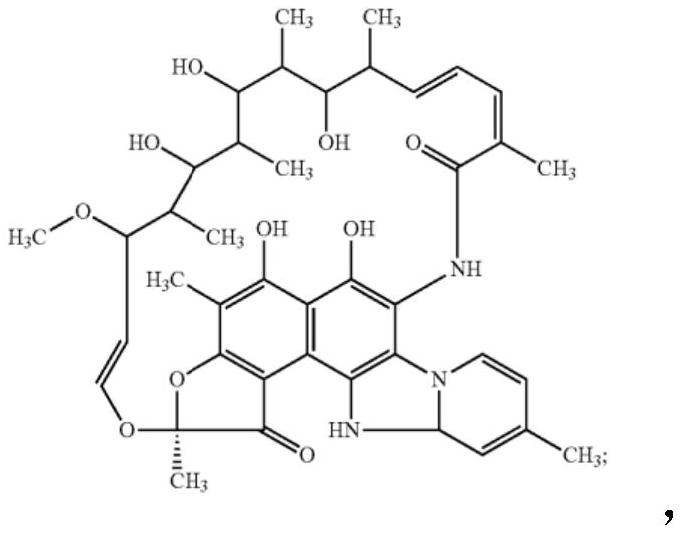
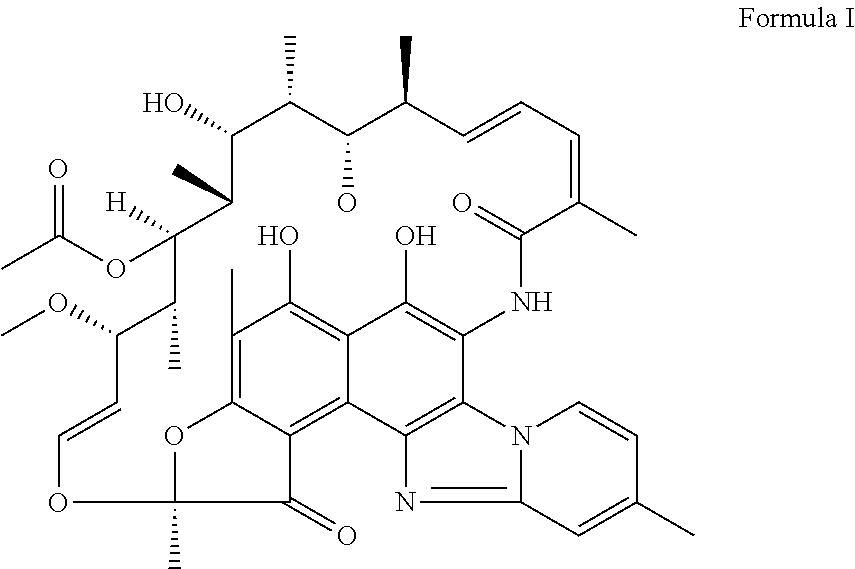
The invention discloses application of a rifamycin-quinolizidone dual-target molecule represented in a formula I in inhibition of ammonia producing floras in gastrointestinal tracts. The rifamycin-quinolizidone dual-target molecule represented in the formula I is similar to an antibacterial spectrum of rifaximin and has relatively strong antibacterial activity to common ammonia producing floras in the gastrointestinal tracts; and meanwhile, the rifamycin-quinolizidone dual-target molecule has the property of low drug resistance frequency and has application prospects in prevention and treatment of infection of hepatic encephalopathy and relevant bacterial genera (types). The formula I is as shown in the specification.
Owner:TENNOR THERAPEUTICS (SUZHOU) LTD
Rifaximin powder, process for preparing the same and controlled release compositions containing said rifaximin useful for obtaining a long-lasting effect
ActiveUS8748447B2Control releaseLasting effectAntibacterial agentsBiocideOral medicationControl release
The present invention describes rifaximin powder and to a process for preparing the same. The invention relates also to a pharmaceutical composition in solid form comprising said rifaximin, pharmaceutically acceptable excipients and optionally other ingredients. The compositions according to the invention are suitable for oral administration and are characterized by producing a controlled release of rifaximin, whereby a long-lasting effect is obtained in a patient.
Owner:ALFASIGMA SPA
Compositions and methods for treating microbiota-related psychotropic conditions and diseases
ActiveCN105307654AFacilitate communicationImprove bowel functionOrganic active ingredientsNervous disorderNervous systemActive agent
In alternative embodiments, the invention provides compositions and methods for treating, ameliorating and preventing various disorders and conditions in mammals, including genetically-predisposed and chronic disorders, where the microbial or bacterial flora of the bowel is at least one causative or symptom-producing factor, for example, where the microbial or bacterial flora of the bowel manufactures neurotoxins or neurotoxic agents that enter the body through the gastrointestinal (Gl) tract, e.g. the colon, and reach the systemic space, e.g., by neural streaming or via the circulation, to reach the central nervous system (CNS), including the brain, the peripheral nervous system (PNS), and other nervous systems. In alternative embodiments, methods and compositions of the invention comprise or comprise use of medications, formulations and pharmaceuticals comprising rifaximin or equivalent active agents that can suppress or eradicate the microbiota super-infection that causes various psychotropic disorders. These compositions have been found to be affective in a broad spectrum of disorders but particularly in the obsessive compulsive disorder group (OCD).
Owner:托马斯·朱利叶斯·波洛迪
Polymorphic form of rifaximin and process for its preparation
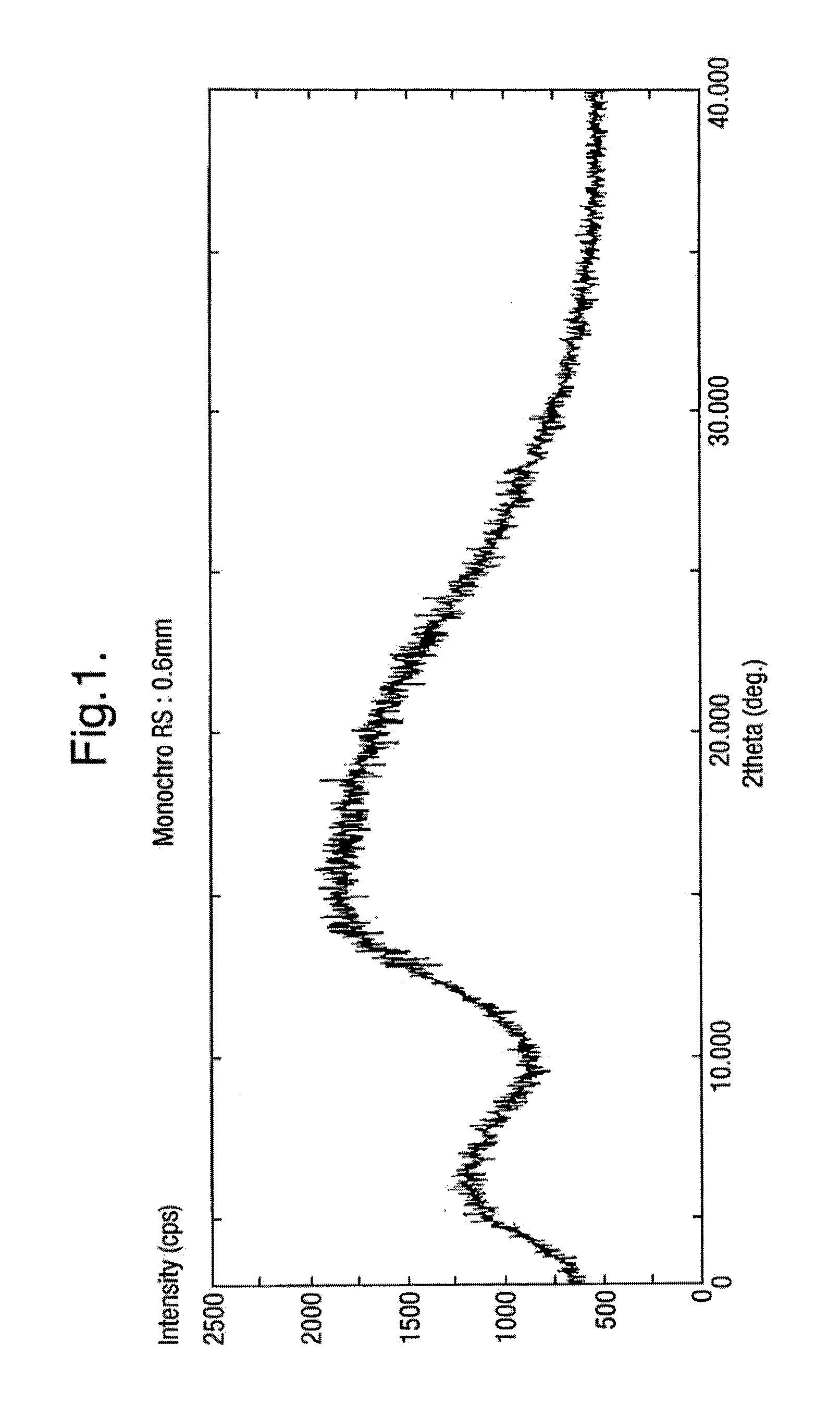
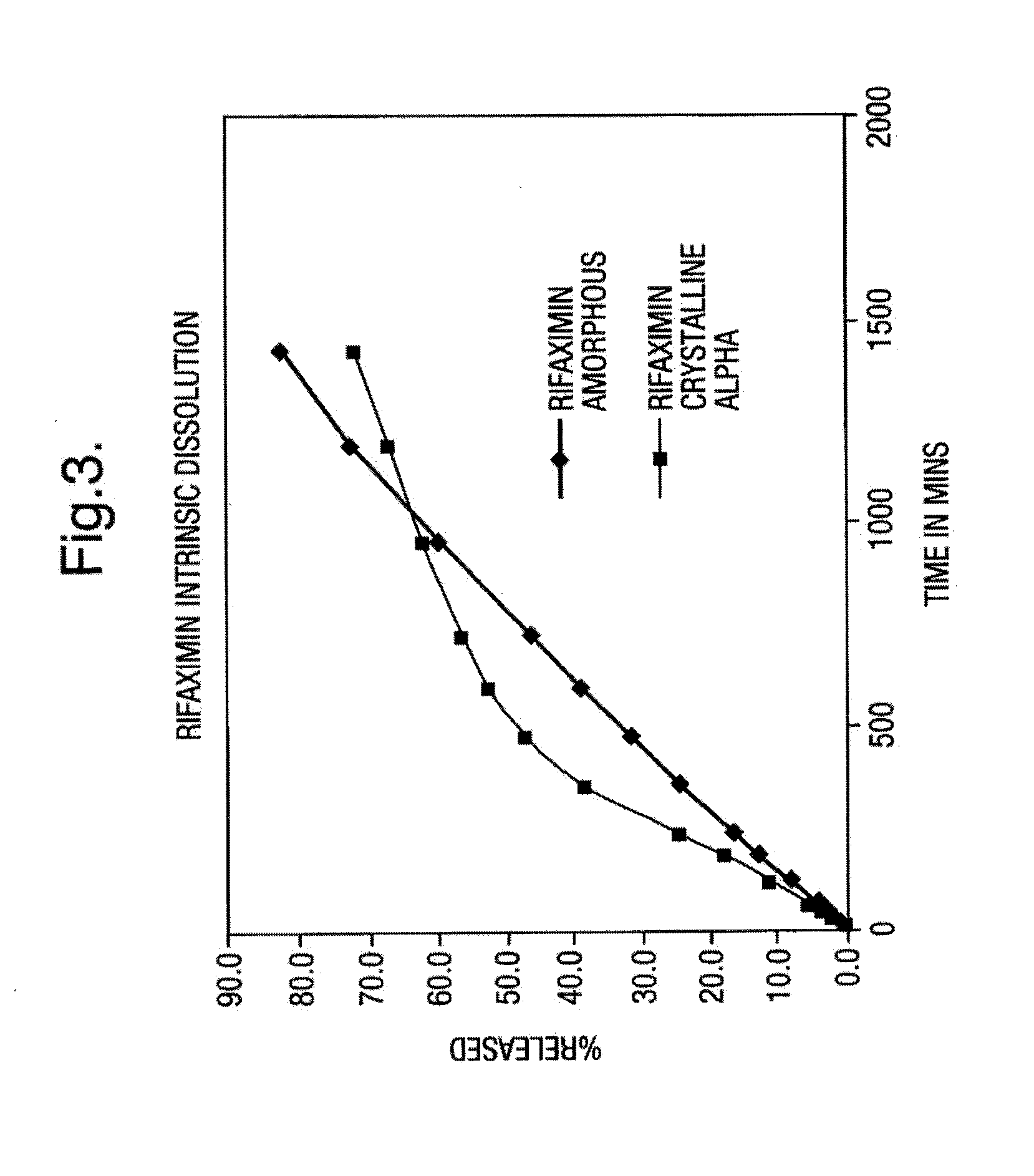
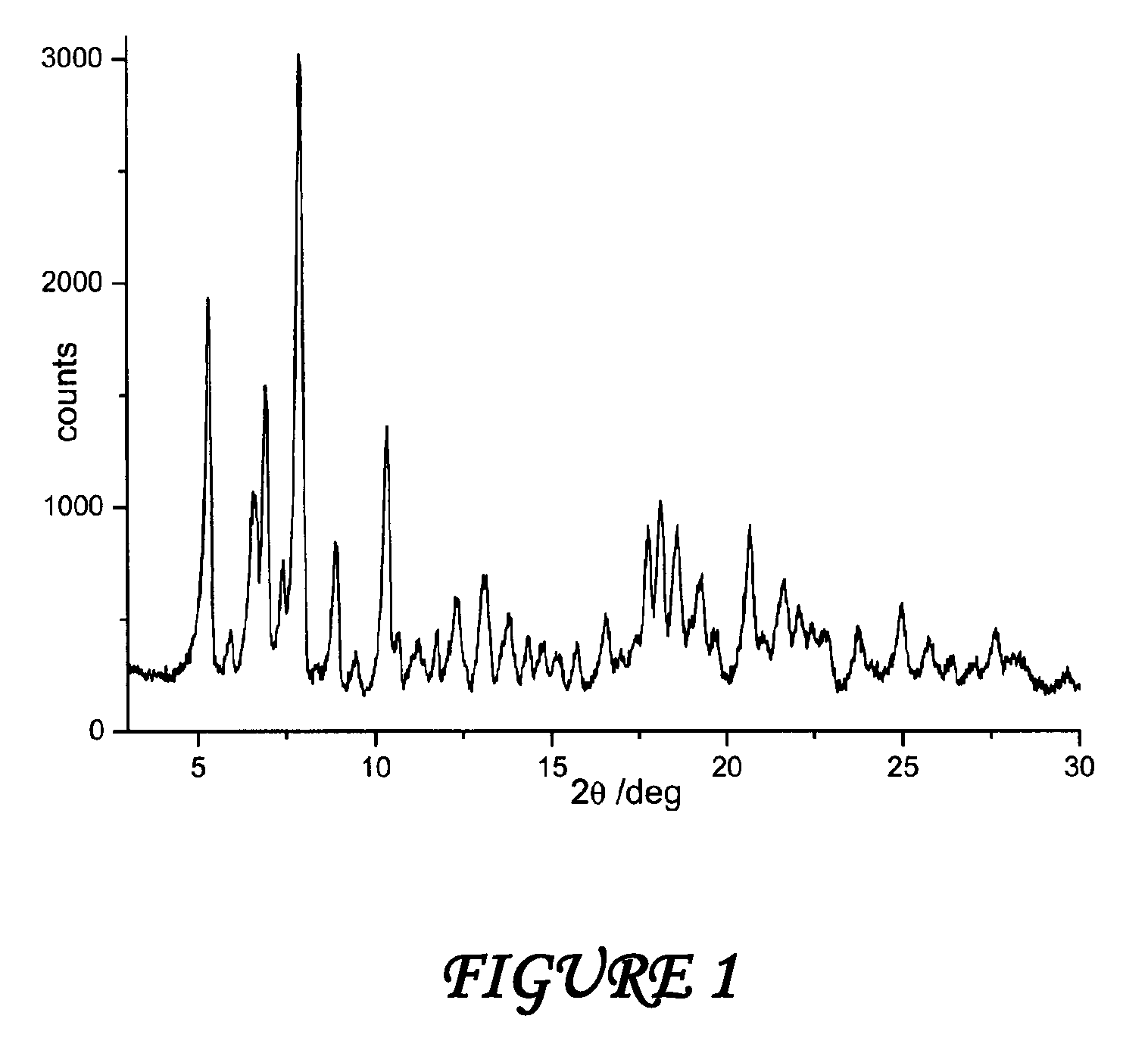
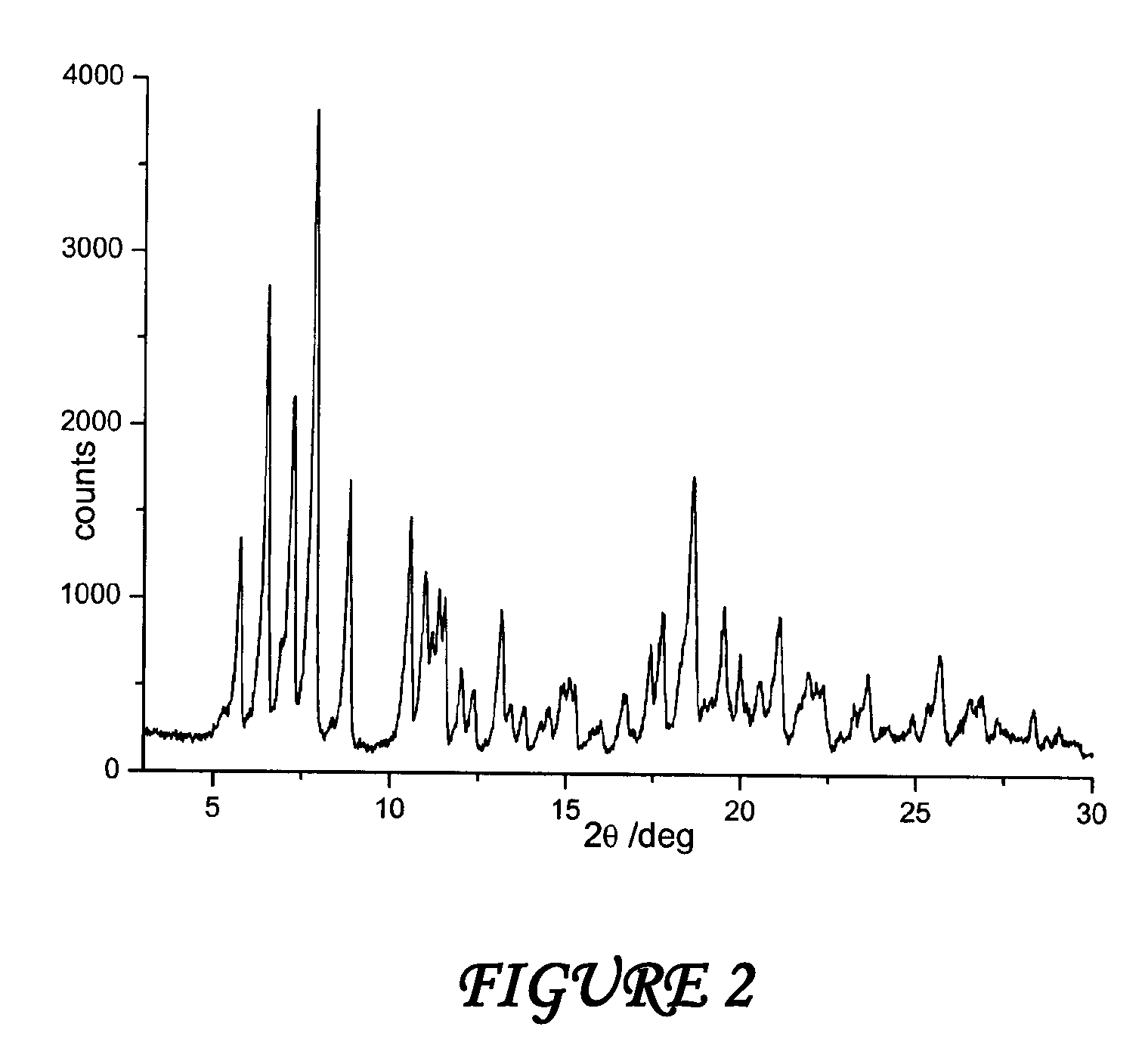
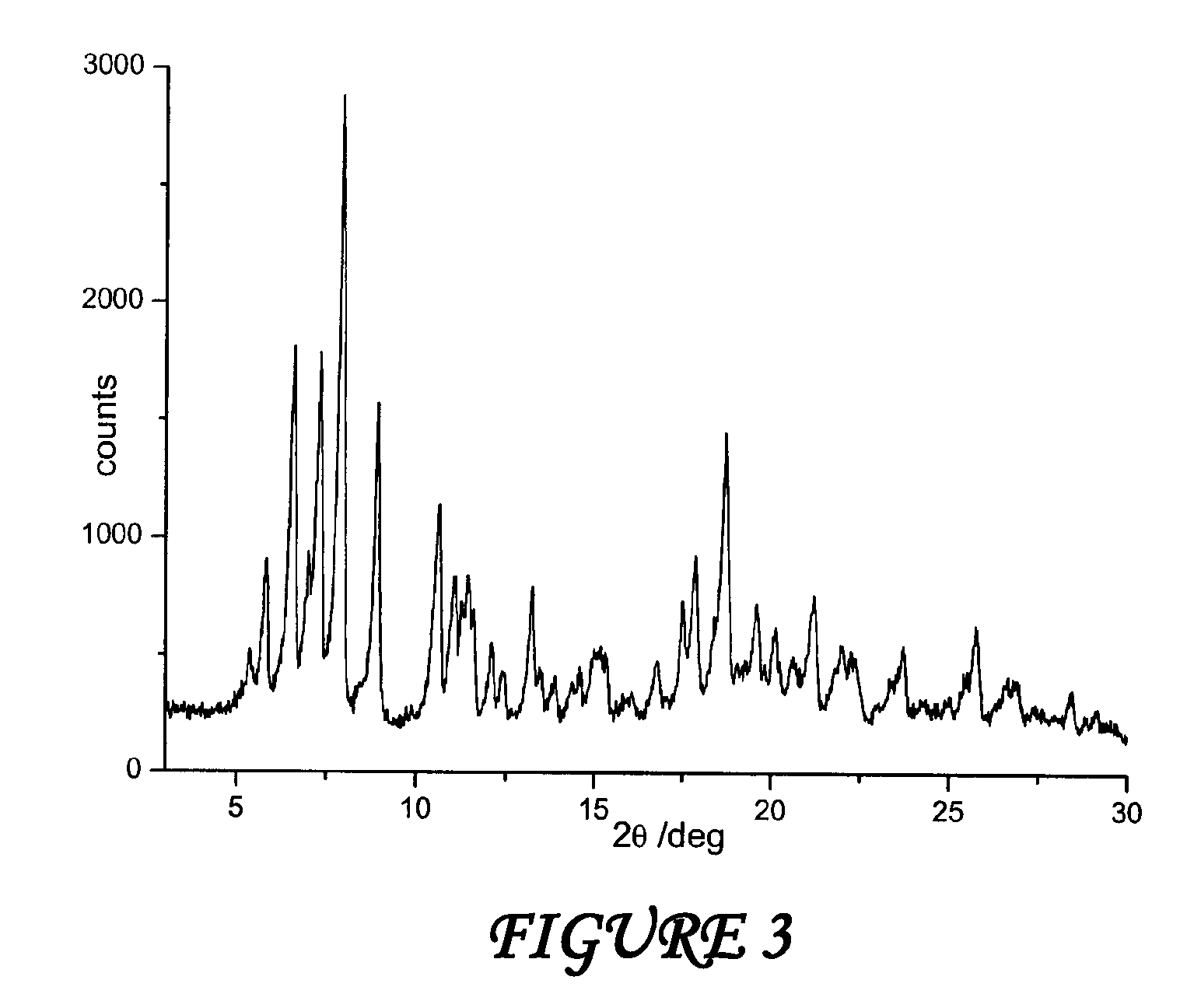
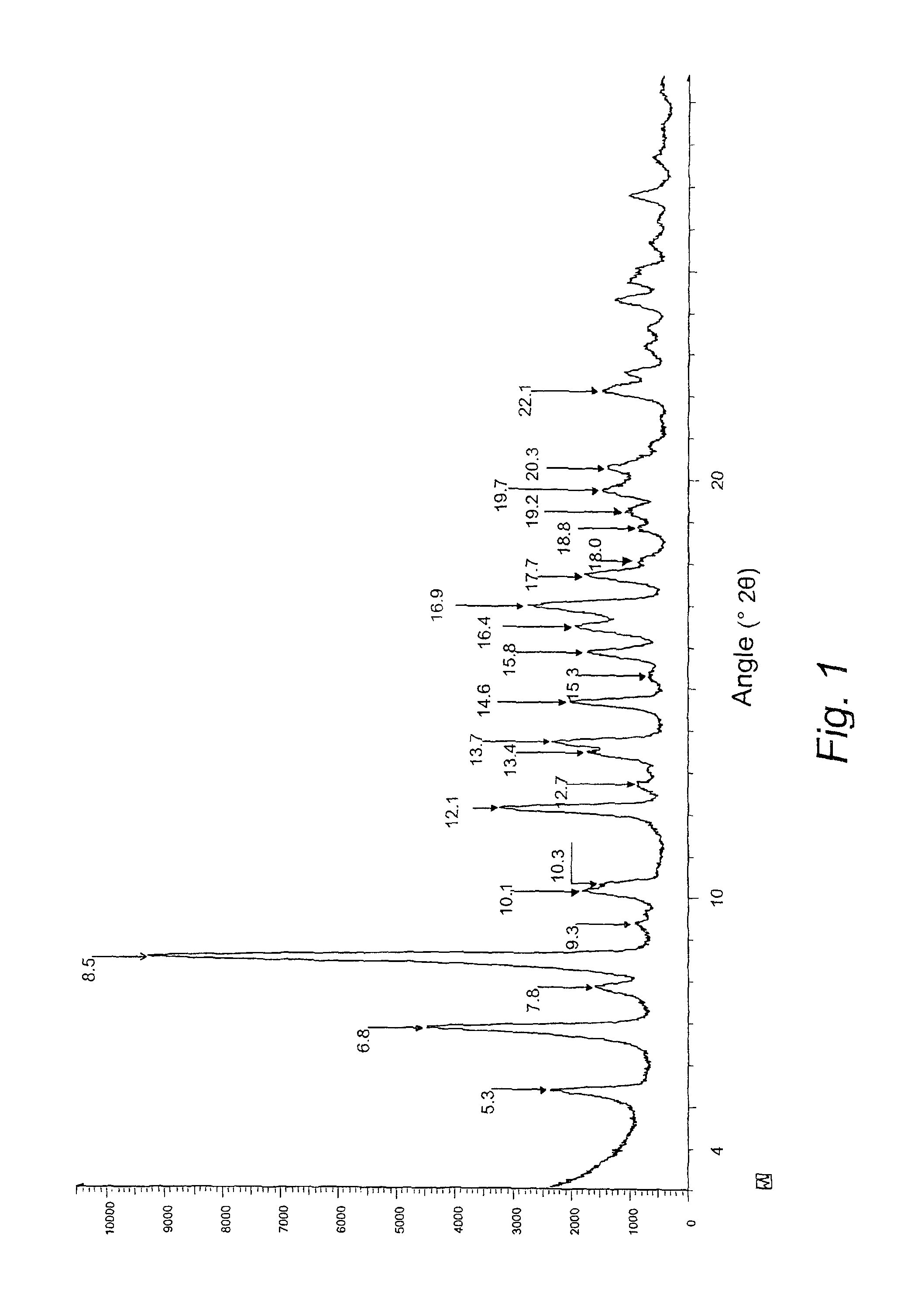
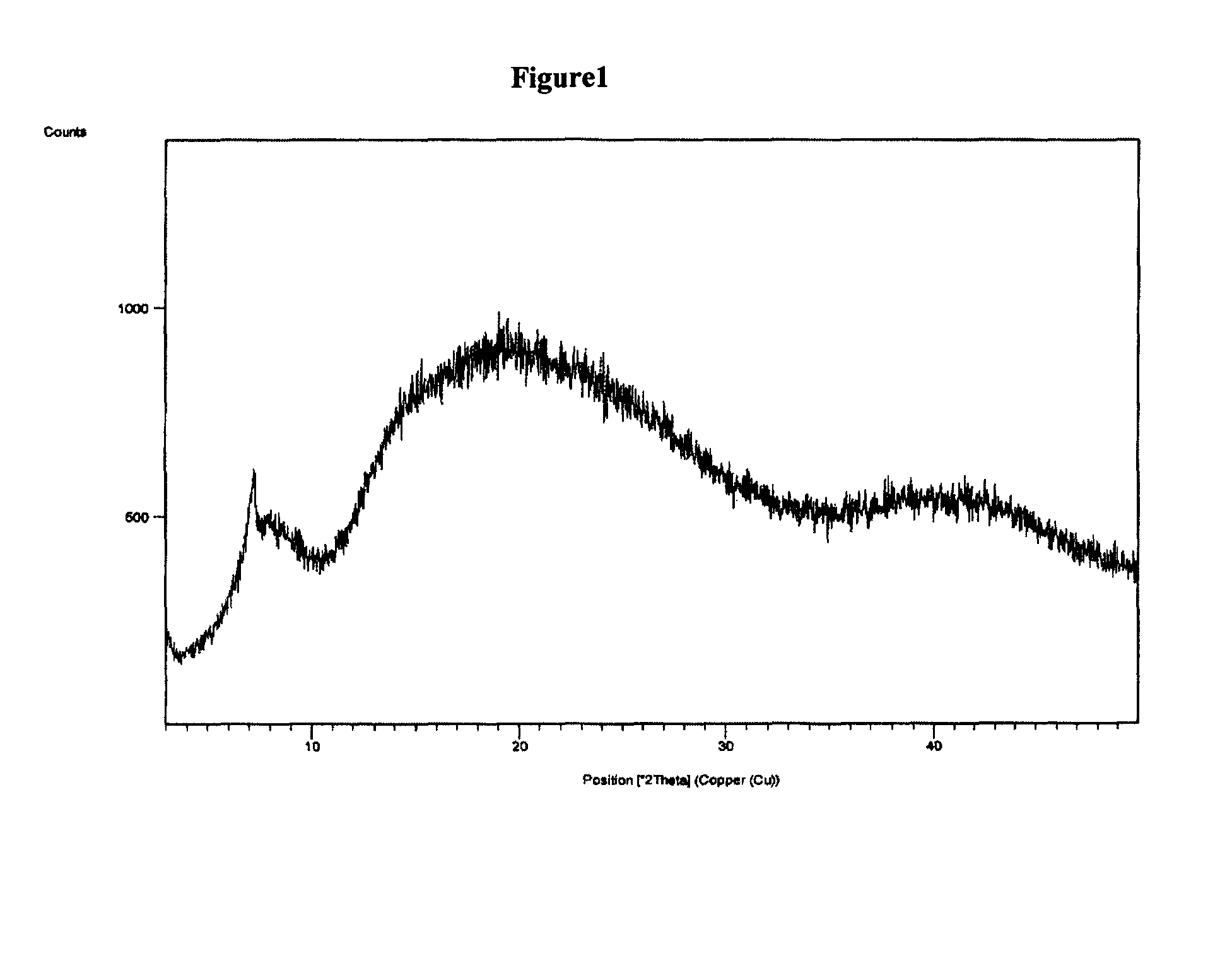
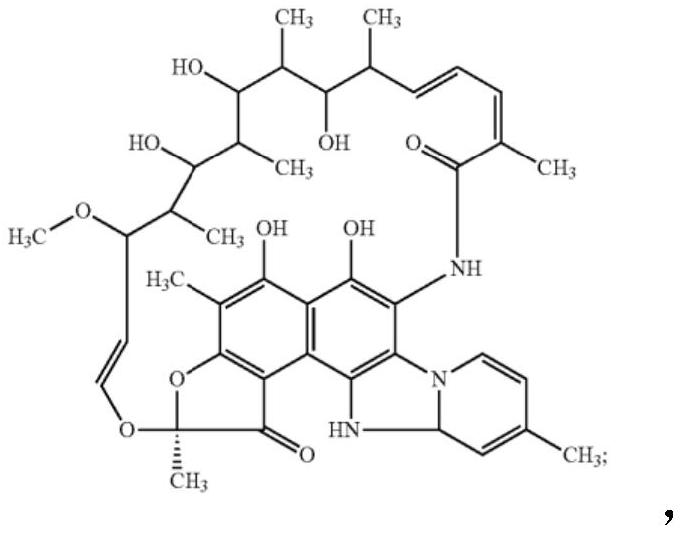
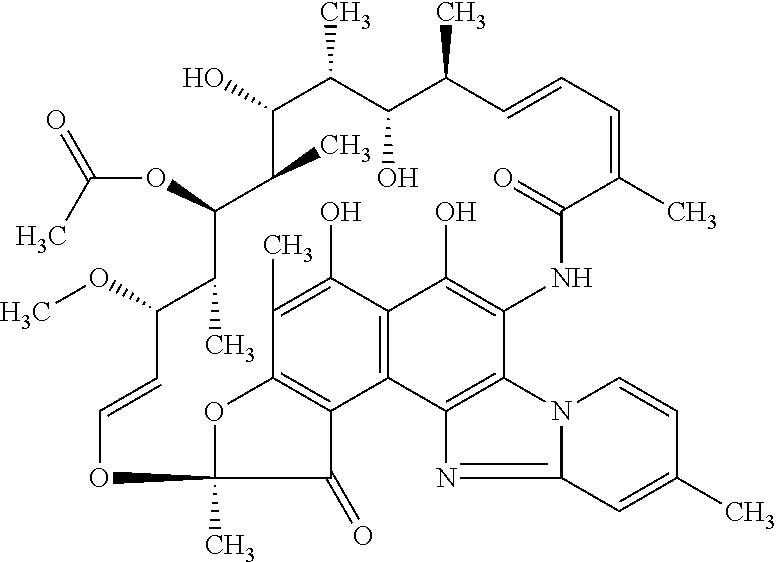
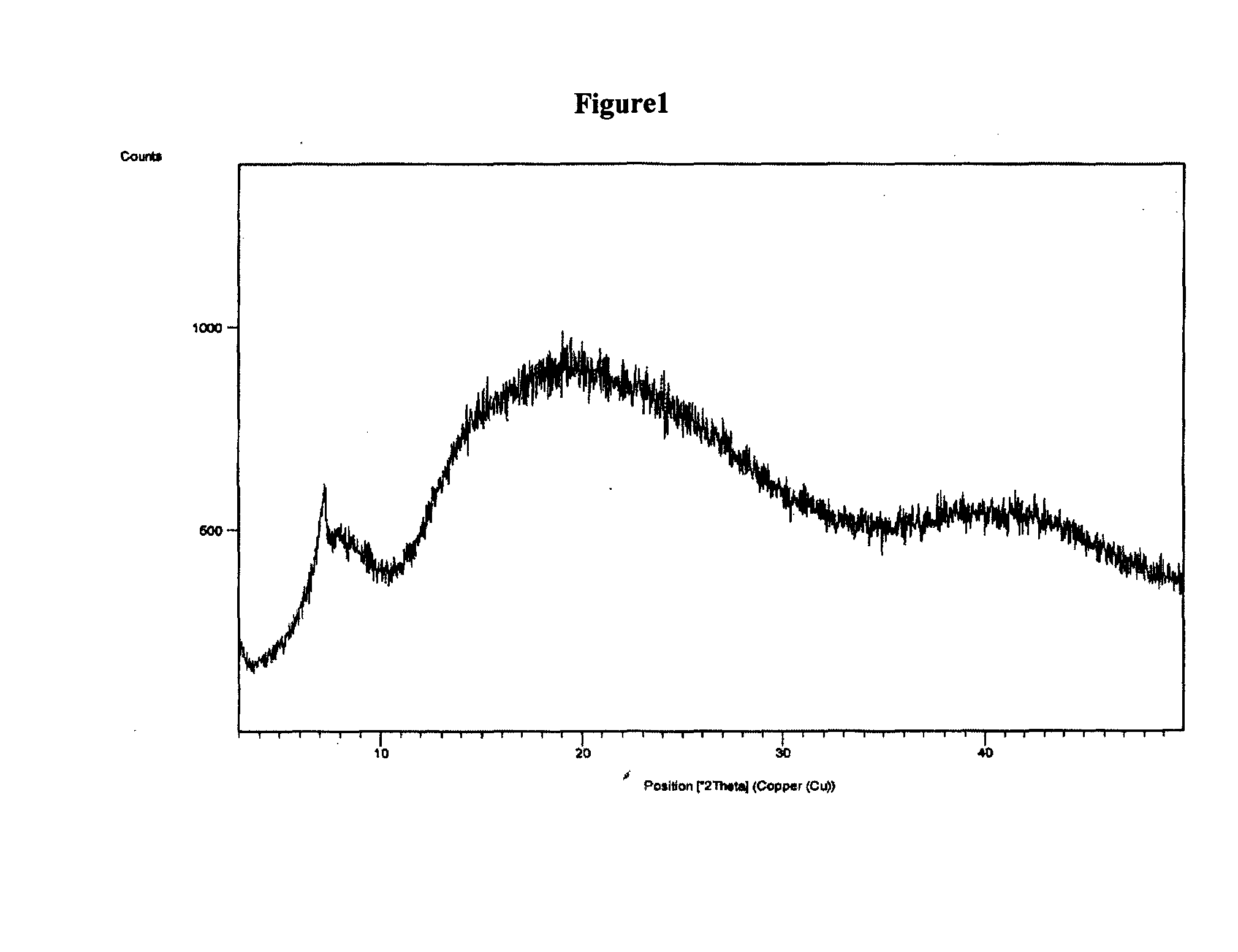
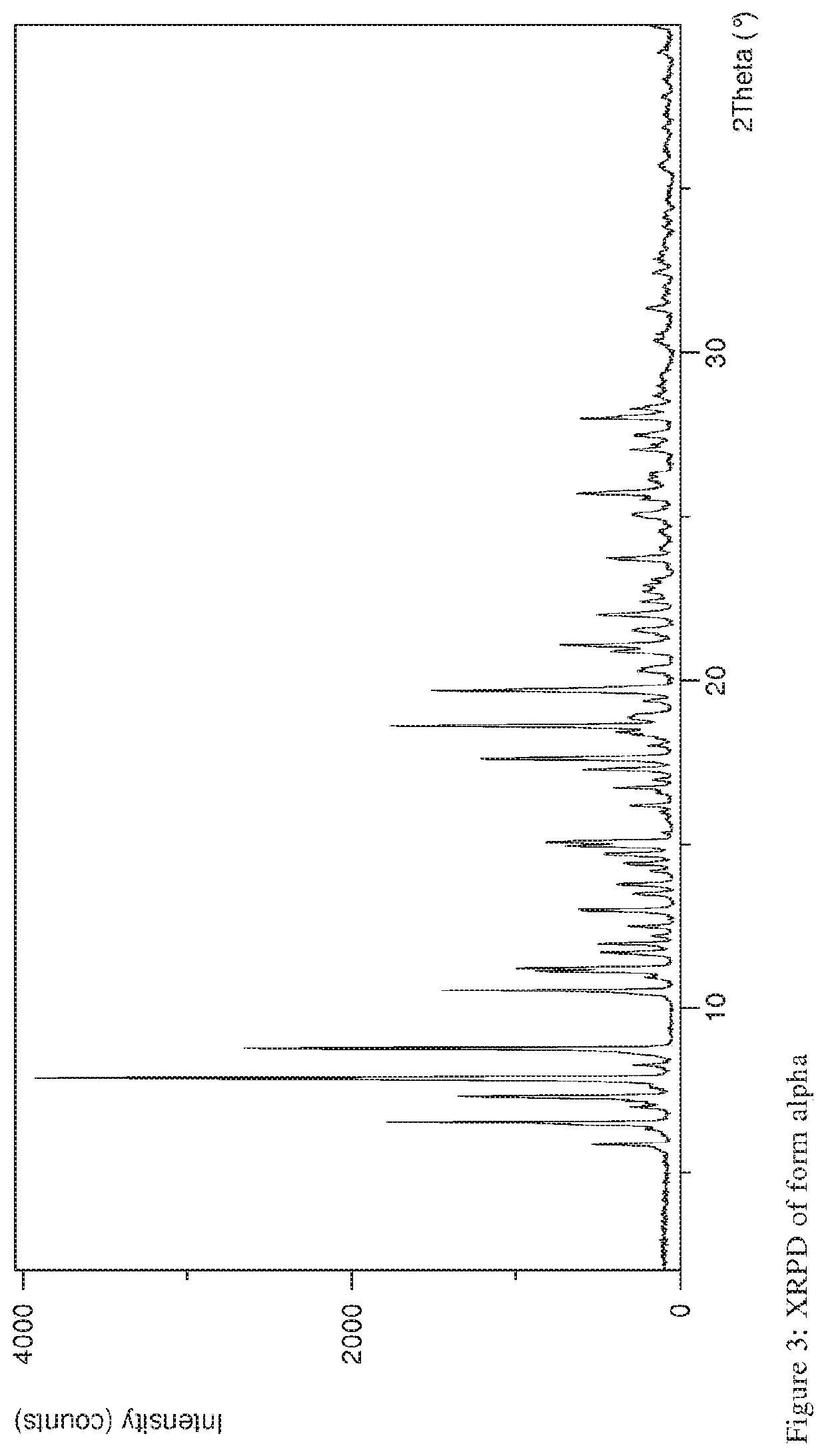
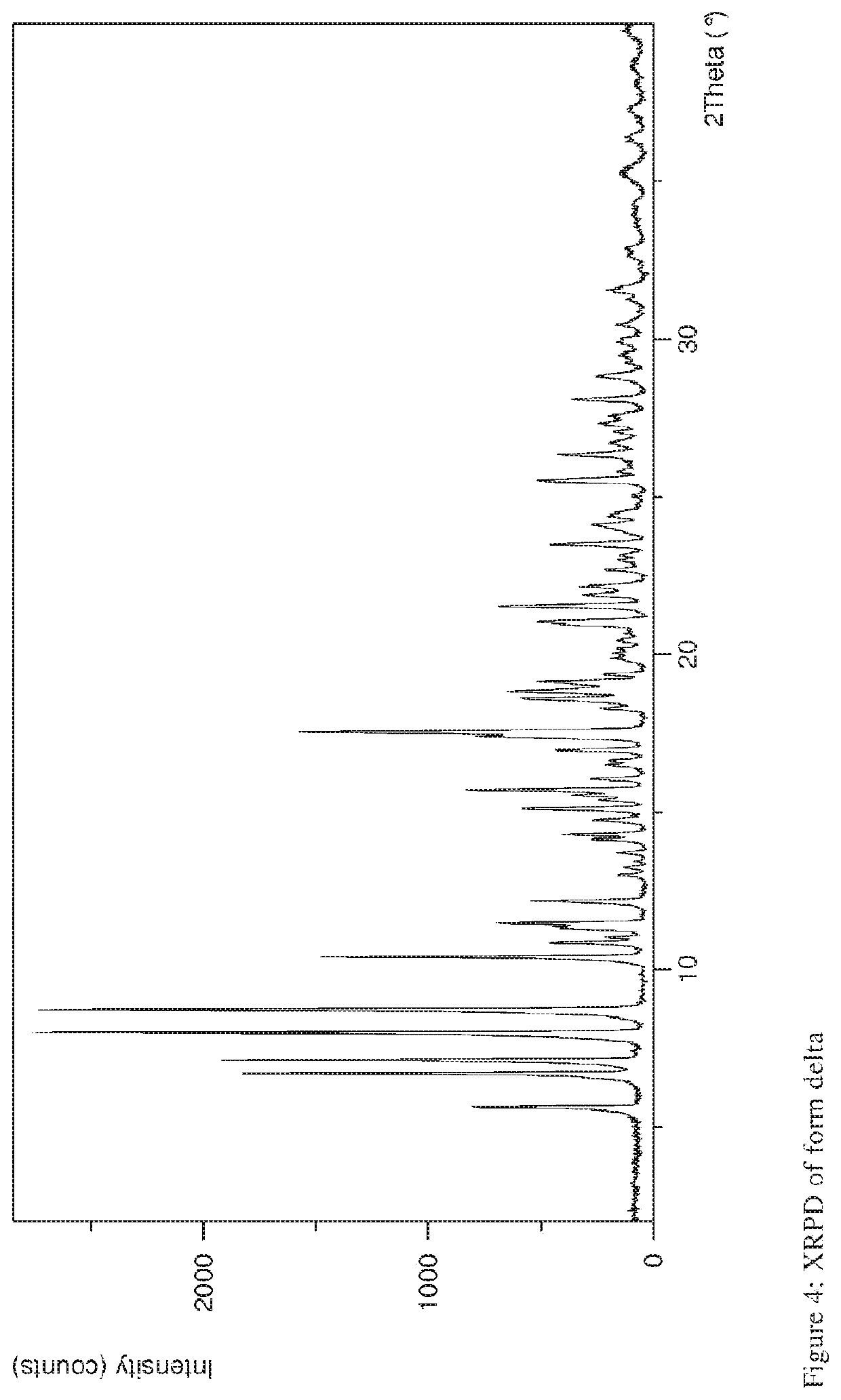
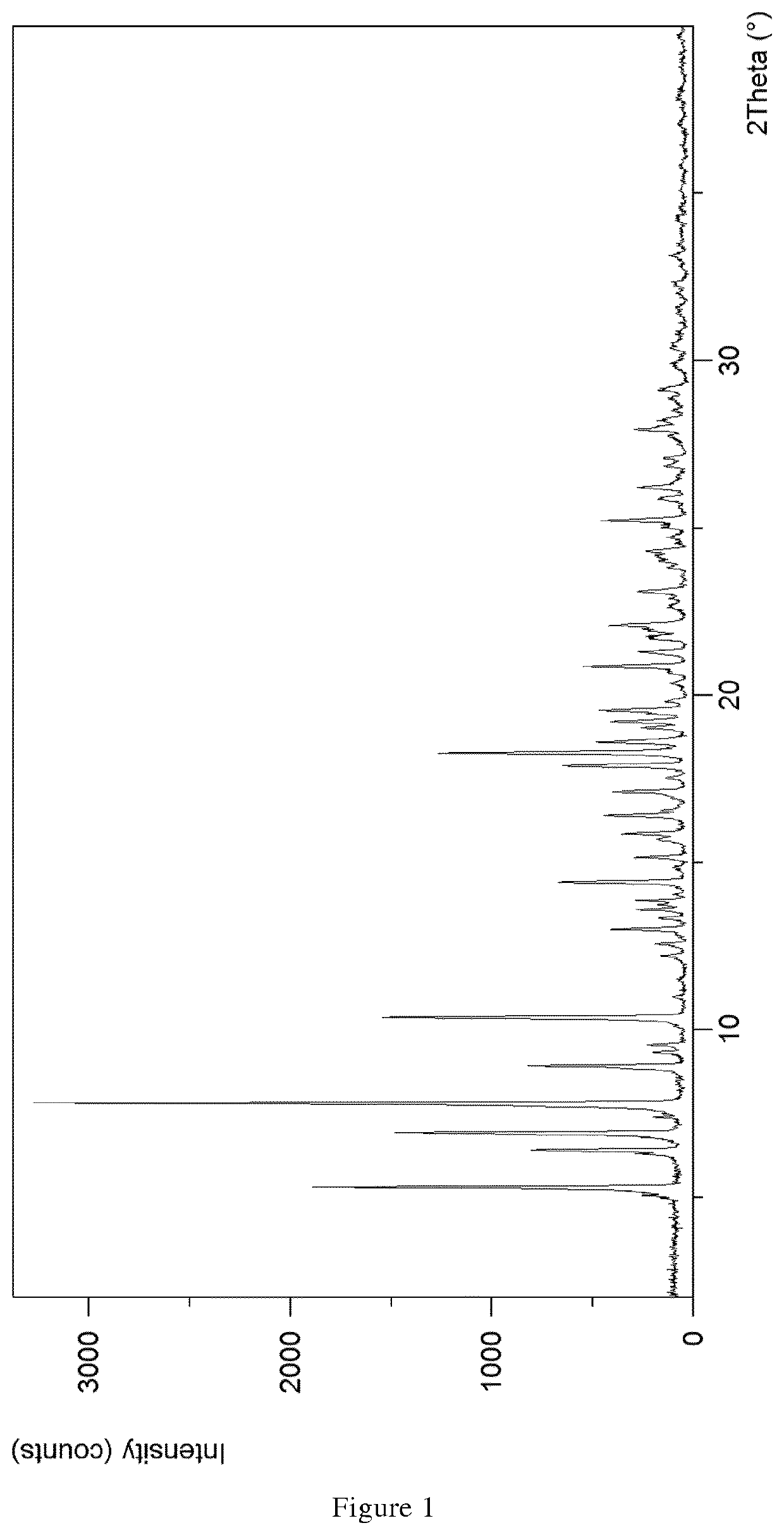
The present invention discloses a stable amorphous form of Rifaximin characterized by having X-ray powder diffraction pattern as given in FIG. 1, having a 2θ peaks at 7.2 and having moisture content in the range of 3% to 4% preferably, 3.4% to 3.7%. This invention also discloses a novel process for its preparation.
Owner:SEQUENT SCI LTD
Pharmaceutical compositions of rifaximin
ActiveUS8383151B2Extended stayImprove complianceAntibacterial agentsBiocideImmediate releasePharmaceutical medicine
A pharmaceutical composition comprising therapeutically effective amount of rifaximin or pharmaceutically acceptable salt or enantiomer or polymorph thereof, pharmaceutically acceptable excipient(s) and release controlling agent(s). Pharmaceutical composition of rifaximin comprising: at least two entities wherein one entity is an immediate release or fast release and the other is controlled release. The pharmaceutical composition in the form of multilayer tablet comprising, at least one layer comprising, therapeutically effective amount of rifaximin or pharmaceutically acceptable salt or enantiomer or polymorph thereof, pharmaceutically acceptable excipient(s); said layer providing controlled release rifaximin; and at least one layer which provides increased residence time of the dosage form in the gastrointestinal tract. The pharmaceutical formulation comprising rifaximin having an in vitro dissolution profile, wherein about 70% of rifaximin is released in about 24 hours. The composition comprising therapeutically effective amount of rifaximin or pharmaceutically acceptable salt(s) or enantiomer(s) or polymorph(s) thereof, one or more release controlling agent(s) and pharmaceutically acceptable excipient(s) causing pathogenic eradication.
Owner:LUPIN LTD
Rifaximin enteric-coated preparation composition and preparation method thereof
InactiveCN101653428AGood curative effectImprove securityAntibacterial agentsOrganic active ingredientsDiseaseSide effect
The invention discloses a rifaximin enteric-coated preparation composition and a preparation method thereof. The rifaximin enteric-coated preparation composition is mainly prepared from a rifaximin bulk drug and proper auxiliary materials. The rifaximin enteric-coated preparation provided by the invention is effective and safe for the improvement on a rifaximin preparation, and has the advantagesof less stimulations to the stomach to reduce side effects and the like compared with a common rifaximin preparation. The rifaximin enteric-coated preparation composition is particularly suitable forpatients with stomach upset diseases and can maximize the contact between an active component rifaximin and intestinal mucus. The invention provides a novel dosage form which is safer and has better curative effect; and the preparation process of the rifaximin enteric-coated preparation composition has good quality controllability and stability.
Owner:北京瑞伊人科技发展有限公司 +1
Rifaximin sustained-release preparation composition and method for preparing the same
InactiveCN101502513AGood controllabilityImprove stabilityOrganic active ingredientsDigestive systemPatient complianceDrug product
The invention discloses a rifaximin sustained-release preparation combination and a preparation method thereof. The rifaximin sustained-release preparation combination is mainly prepared from rifaximin bulk drugs, sustained-release materials and other appropriate auxiliary materials. The rifaximin sustained-release preparation provided by the invention can deaccelerate the release rate of the main drugs, reduce the frequency of administration and improve the patient compliance. The invention provides a novel form of drug having better patient compliance and having the advantages of high quality controllability and stability of the preparation process.
Owner:山东淄博新达制药有限公司
Pharmaceutical Composition Containing Polymyxin B/Trimethoprim based Therapeutics
ActiveUS20190175550A1Increase virulenceAntibacterial agentsOrganic active ingredientsRifabutinTrimethoprim
The present invention features an antibacterial composition comprising 1) a composition A comprising polymyxin B and trimethoprim; and 2) an antibiotic agent selected from the group consisting of rifampicin, rifabutin, rifapentine, rifaximin, pefloxacin mesylate, sparfloxacin, sarafloxacin HCl, tobramycin, lomefloxacin, besifloxacin, danofloxacin mesylate, enrofloxacin, nadifloxacin and clinafloxacin, a topical pharmaceutical thereof, and a method of treating bacterial infections using mixtures of 1 and 2.
Owner:UNIVERSITY OF ROCHESTER
Rifaximin hapten and rifaximin artificial antigen, and preparation methods and application thereof
InactiveCN111560027AThe synthesis method is simpleHigh purityOvalbuminSerum albuminAntiendomysial antibodiesPharmaceutical drug
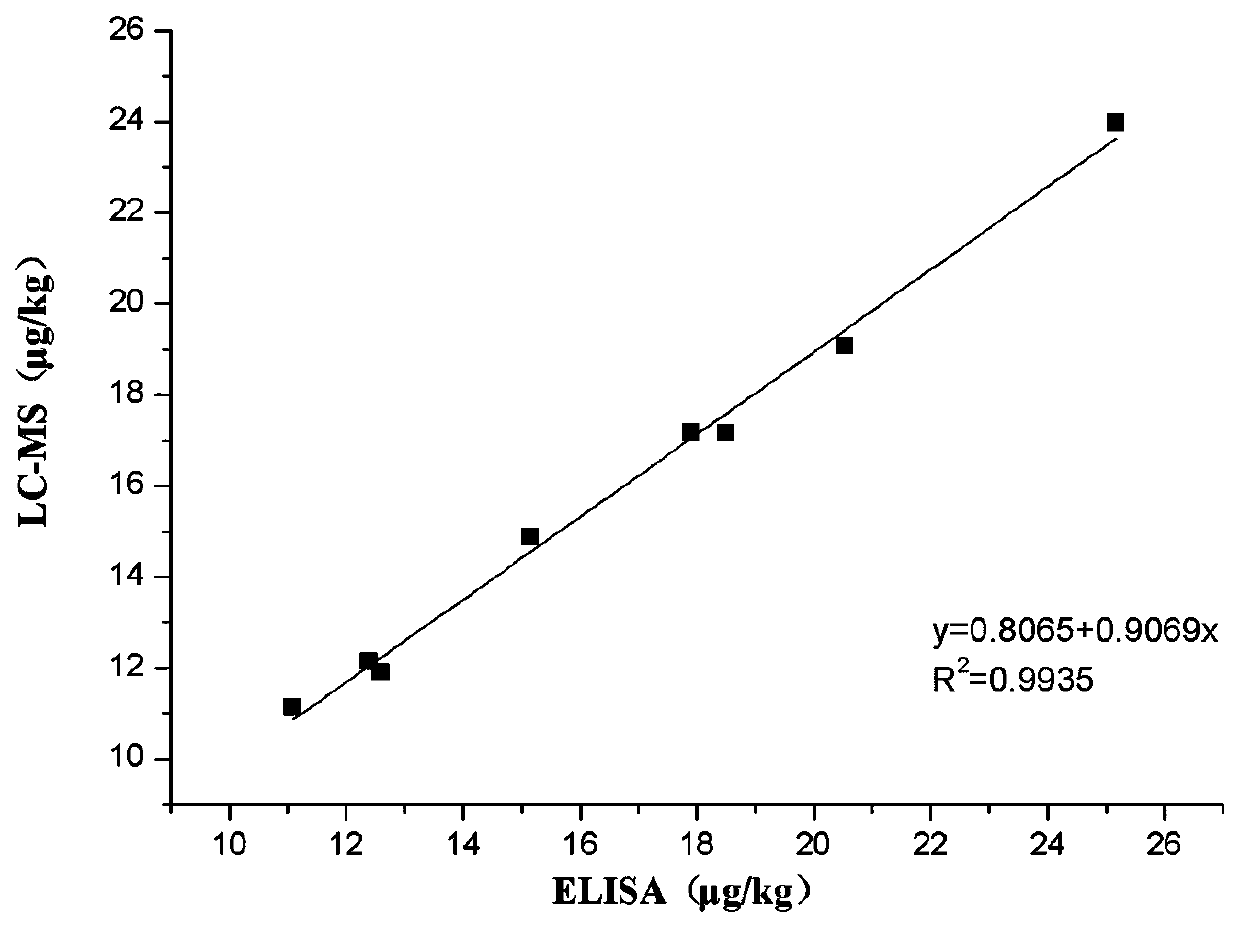
The invention discloses a rifaximin hapten and a rifaximin artificial antigen, and a preparation method and application of the rifaximin hapten and the rifaximin artificial antigen. The rifaximin artificial antigen provided by the invention is an antigen obtained by coupling the rifaximin hapten shown as a formula I which is described in the specification with a carrier protein. The rifaximin artificial antigen provided by the invention is simple in synthesis method, high in purity and high in yield, and has important value for preparation of a rifaximin antibody and detection of rifaximin drug residues.
Owner:北京维德维康生物技术有限公司
Rifaximin anti-rectal dysfunction preparation
Owner:SALIX PHARMA INC
Compositions and methods for treating inflammatory bowel disease and fusobacteria-caused or related diseases and conditions
Provided herein are pharmaceutical compositions, therapeutic combinations, devices and methods for treating, ameliorating, reversing, causing the remission of, and / or preventing (acting as a prophylaxis, or preventing the initiation of) an inflammatory bowel disorder (IBD) or inflammatory bowel disease (IBD), Ulcerative Colitis; Crohn's disease; J-pouch; fistulising Crohn's disease; a Colitis which can be microscopic, lymphocytic or collagenous; an eosinophilic colitis; indeterminate colitis; idiopathic colitis; diverticulosis and diverticulitis; relapsing diverticulitis; constipation associated inflammatory bowel disease and / or small intestinal bacterial overgrowth; Irritable Bowel Syndrome (IBS) with or without diarrhoea, constipation or pain predominant IBS; periodontitis; rheumatoid arthritis; respiratory infections, appendicitis, vascular disorders such as thrombophlebitis; bacteremia; osteomyelitis; septic shock; Alzheimer's disease; Lemierre syndrome (postanginal sepsis); colonic polyps or adenomas (optionally hyperplastic, adenomatous or serrated adenomas) or preventing the growth of colonic polyps or adenomas, bowl cancer, or metastases (optionally preventing the initiation or promotion of bowl cancer or metastasis); pharyngitis; otitis; sinusitis; and any disease, symptom or condition caused or exacerbated by a Fusobacteria (optionally, a F. nucleatum or F. varium) infection. In alternative embodiments, pharmaceutical compositions comprise rifaximin alone or in combination with other antibiotics or drugs.
Owner:托马斯·朱利叶斯·波洛迪
Pharmaceutical Composition
InactiveUS20130315988A1Improve solubilityIncrease surface areaAntibacterial agentsPowder deliveryMedicineMicrometre
A composition comprising rifaximin in the form of particles, wherein substantially all the particles have a particle size less than or equal to 2 micrometres.
Owner:CIPLA LTD
Compositions, devices and methods for treating autism
The present invention relates to a method for treating, ameliorating, reversing and / or preventing autism or an autism spectrum disorder (ASD) via the administration of a formulation, a pharmaceuticalpreparation or a pharmaceutical composition comprising or consisting of: (a) a rifaximin (optionally a XIFAXAN, XIFAXANTA or NORMIXT), an extended intestinal release (EIR) rifaximin, a rifamycin derivative, a rifampicin (or rifampin) (optionally RIFADIN), a rifabutin (optionally MYCOBUTIN), a rifapentine (optionally PRIFTIN), a rifalazil, a bicozamycin, or a mixture or combination thereof, or (b)a rifaximin (optionally a XIFAXAN, XIFAXANTA or NORMIX) and at least one additional antimicrobial or antibiotic agent, wherein optionally the at least one additional antimicrobial or antibiotic agentcomprises a vancomycin, a metronidazole (optionally FLAGYLTM, METROTM), a tinidazole (optionally FASIGYN, SIMPLOTAN, TINDAMAX) or a combination thereof
Owner:托马斯·朱利叶斯·波洛迪
Novel polymorphic form of rifaximin and process for its preparation
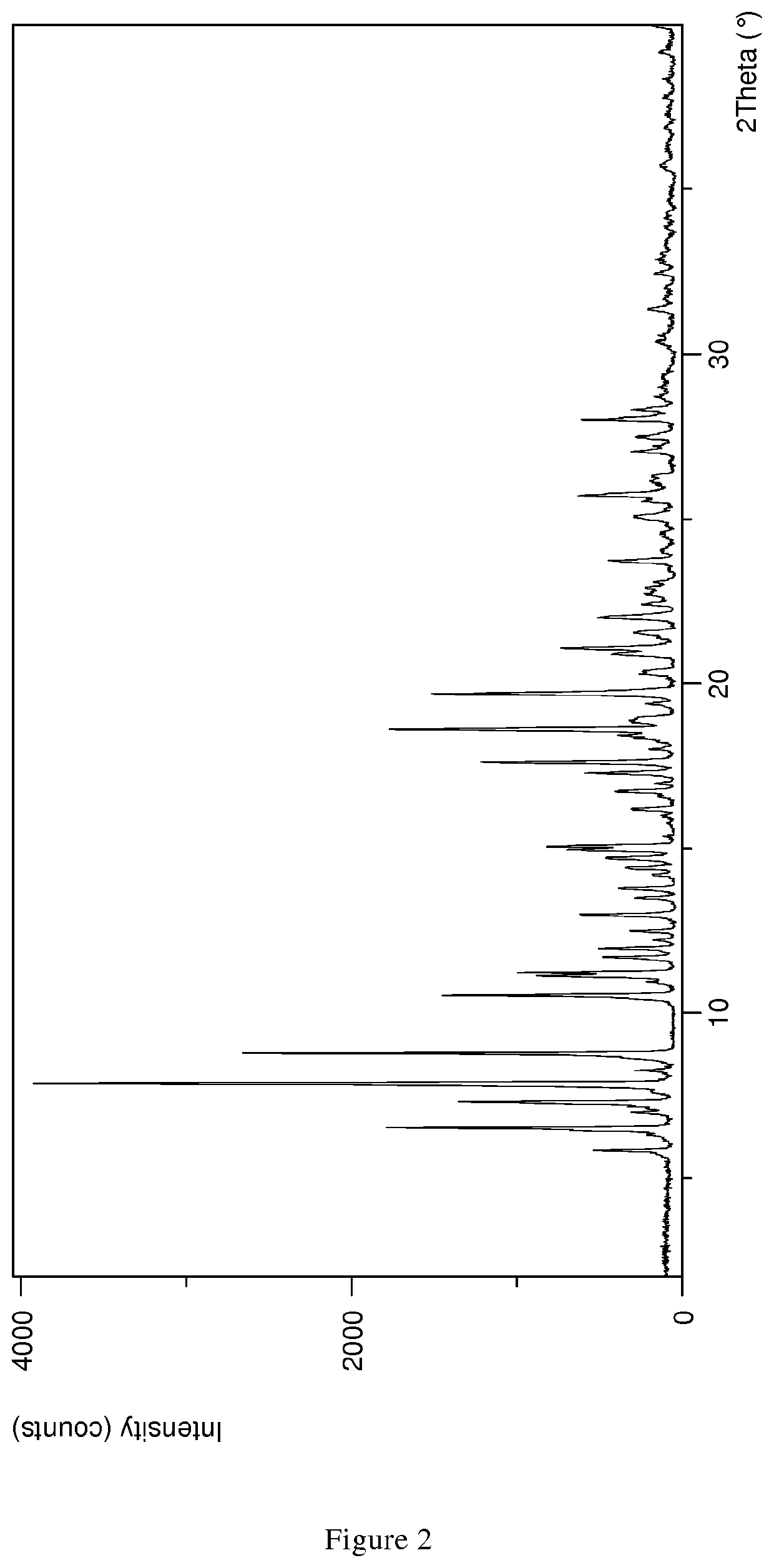
The present invention discloses a stable amorphous form of Rifaximin characterised by having X-ray powder diffraction pattern as given in FIG. 1, having a 2θ peaks at 7.2 and having moisture content in the range of 3% to 4% preferably, 3.4% to 3.7%. This invention also discloses a novel process for its preparation.
Owner:SEQUENT SCI LTD
Synthesis process of rifaximin-D6
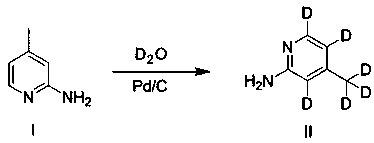
InactiveCN111423456AShort stepsEasy to operateIsotope introduction to heterocyclic compoundsPalladium on carbonMethyl palmoxirate
The invention provides a synthesis process of rifaximine-D6, which comprises the following steps: 1) by using compounds I2-amino-4-methylpyridine and D2O as raw materials, reacting in the presence of5wt% palladium on carbon and under the protection of nitrogen to obtain a deuterated intermediate, i.e., a compound II 4-methyl-2-aminopyridine-D6; step 2), dissolving rifamycin S and the deuterated intermediate compound II 4-methyl-2-aminopyridine-D6 prepared in the step 1) into dichloromethane; under the protection of nitrogen, stirring at room temperature, dropwise adding a dichloromethane solution dissolved with elemental iodine, reacting at room temperature overnight for 18 hours, dropwise adding an L-ascorbic acid aqueous solution, stirring, and after the reaction is completed, washing and purifying to obtain the product rifaximin-D6. The synthesis method has the following technical effects that the synthesis method of the intermediate II of rifaximin-D6 is simple and convenient, andraw materials are easy to obtain; the steps for synthesizing rifaximin-D6 are simple and short, and the operation is easy.
Owner:南京昊绿生物科技有限公司
Rifaximin enteric sustained-release preparation composition and method for preparing the same
InactiveCN101502514ASlow release rateReduce the frequency of takingOrganic active ingredientsDigestive systemPatient complianceIrritation
The invention discloses a rifaximin enteric sustained-release preparation combination and a preparation method thereof. The rifaximin enteric sustained-release preparation combination is mainly prepared from rifaximin bulk drugs, medicinal sustained-release and enteric materials and other appropriate auxiliary materials. The rifaximin enteric sustained-release preparation provided by the invention can not only prevent rifaximin from disintegrating in the stomach and causing irritation to gastric mucosa and avoid the adverse reactions caused by the administration, such as nausea, abdominal pain, diarrhea and the like, but also deaccelerate the release rate of drugs, reduce the frequency of administration and improve the patient compliance. The invention provides a novel form of drug having higher safety and better patient compliance and having the advantages of high quality controllability and stability of the preparation process.
Owner:山东淄博新达制药有限公司
Novel veterinary uterus injectant as well as preparation method and application thereof
ActiveCN113842358AImprove stabilityIncrease infectionAntibacterial agentsOrganic active ingredientsFreeze-dryingVeterinary Drugs
The invention provides a preparation method of a novel veterinary uterus injectant, which belongs to the field of veterinary preparations, and comprises the following steps: respectively mixing an adsorption carrier with rifaximin self-microemulsion and a growth repair factor to prepare self-microemulsion drug-loaded particles and growth repair factor drug-loaded particles; and mixing a first matrix, a second matrix and water for injection to prepare an injection matrix, mixing the injection matrix with the self-microemulsion drug-loaded particles and the growth repair factor drug-loaded particles, and freeze-drying to prepare the uterus injectant, by utilizing the cell growth promoting effect of the growth repair factor and the cell proliferation microenvironment provided by the gel matrix, the repair of endometrial epidermis cells is accelerated, the regeneration of damaged tissues is promoted, and a protective barrier is established, so that new infection in the uterus can be prevented, and the morbidity of endometritis is reduced.
Owner:FOSHAN NANHAI EASTERN ALONG PHARMA CO LTD
Rifaximin liquid preparation
PendingCN114401721AAntibacterial agentsOrganic active ingredientsPharmaceutical drugOrganic chemistry
Provided herein are pharmaceutical compositions that enhance the intestinal level of soluble rifaximin, formulations comprising the compositions, and their use in the treatment of one or more intestinal related disorders.
Owner:バウシュ ヘルス アイルランド リミテッド
Rifaximin-containing preparation for preventing and treating dairy cow mastitis during dry period and method for preparing rifaximin-containing preparation
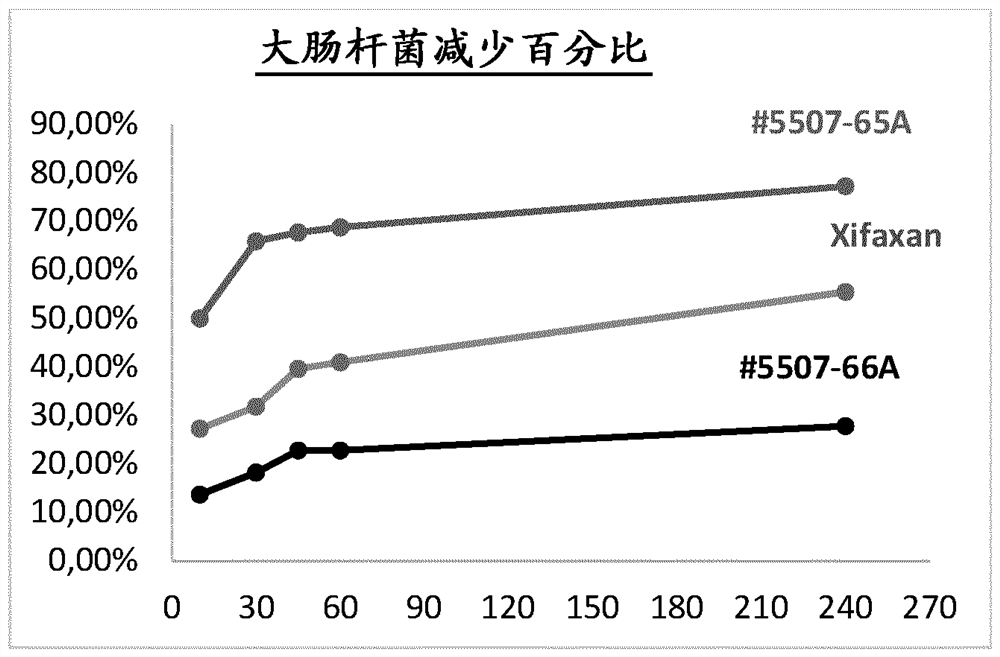
ActiveCN102716168BBroad spectrum antibacterialProlong the action timeAntibacterial agentsOrganic active ingredientsBiotechnologyBacteroides
The invention relates to a rifaximin-containing preparation for preventing and treating dairy cow mastitis during the dry milk period, which comprises the main active ingredients of rifaximin and dandelion plant extracts, the auxiliary ingredients of thickening agent poloxamer 407, a viscosity modifier isopropyl myristate, an antioxidant butylated hydroxytoluene (BHT) and a solvent medical castor oil. The rifaximin-containing preparation can be used as an udder injectant for preventing and treating the dairy cow mastitis during the dry milk period, which is caused by staphylococcus aureus, staphylococcus epidermidis, streptococcus, enterococcus, corynebacterium, Escherichia coli, salmonella, shigella and bacteroides.
Owner:QILU ANIMAL HEALTH PROD
Compositions, devices and methods for treating obsessive-compulsive disorder
In alternative embodiments, provided are pharmaceutical compositions and methods for treating, ameliorating, reversing and / or preventing (acting as a prophylaxis) an Obsessive-Compulsive Disorder (OCD), with or without an accompanying autism or an autism spectrum disorder (ASD), e.g., a regressive autism. In alternative embodiments, these pharmaceutical compositions and methods are dosaged and administered to children in need thereof. In alternative embodiments, pharmaceutical compositions and methods are dosaged, formulated and dosaged as solid, liquid or aerosol preparations or formulations. In alternative embodiments, pharmaceutical compositions comprise rifaximin as the sole antibiotic, or rixafimin and other antimicrobial or antibiotic agent, for example, vancomycin, metronidazole, tinidazole, secnidazole or a combination thereof. As there are various molecular forms of rifaximins, all these are useful and used in methods and compositions as provided herein
Owner:CENT FOR DIGESTIVE DISEASES PTY LTD
Compositions, devices and methods for treating obsessive-compulsive disorder
In alternative embodiments, provided are pharmaceutical compositions and methods for treating, ameliorating, reversing and / or preventing (acting as a prophylaxis) an Obsessive-Compulsive Disorder (OCD), with or without an accompanying autism or an autism spectrum disorder (ASD), e.g., a regressive autism. In alternative embodiments, these pharmaceutical compositions and methods are dosaged and administered to children in need thereof. In alternative embodiments, pharmaceutical compositions and methods are dosaged, formulated and dosaged as solid, liquid or aerosol preparations or formulations. In alternative embodiments, pharmaceutical compositions comprise rifaximin as the sole antibiotic, or rixafimin and other antimicrobial or antibiotic agent, for example, vancomycin, metronidazole, tinidazole, secnidazole or a combination thereof. As there are various molecular forms of rifaximins, all these are useful and used in methods and compositions as provided herein
Owner:CENT FOR DIGESTIVE DISEASES PTY LTD
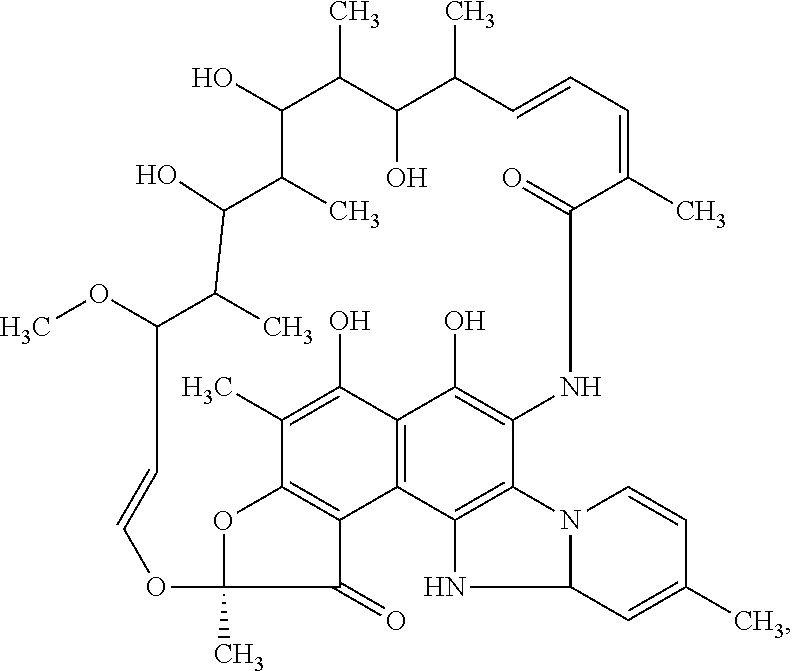
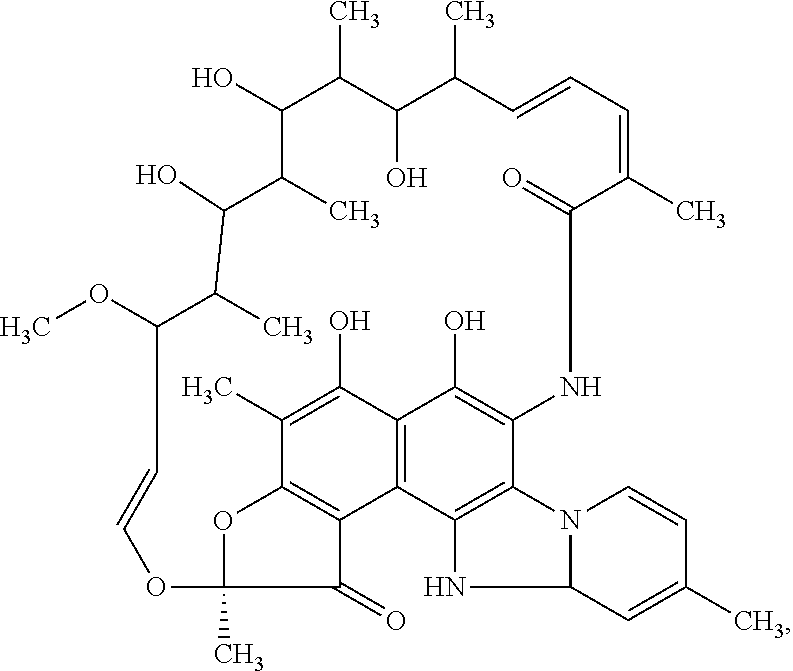
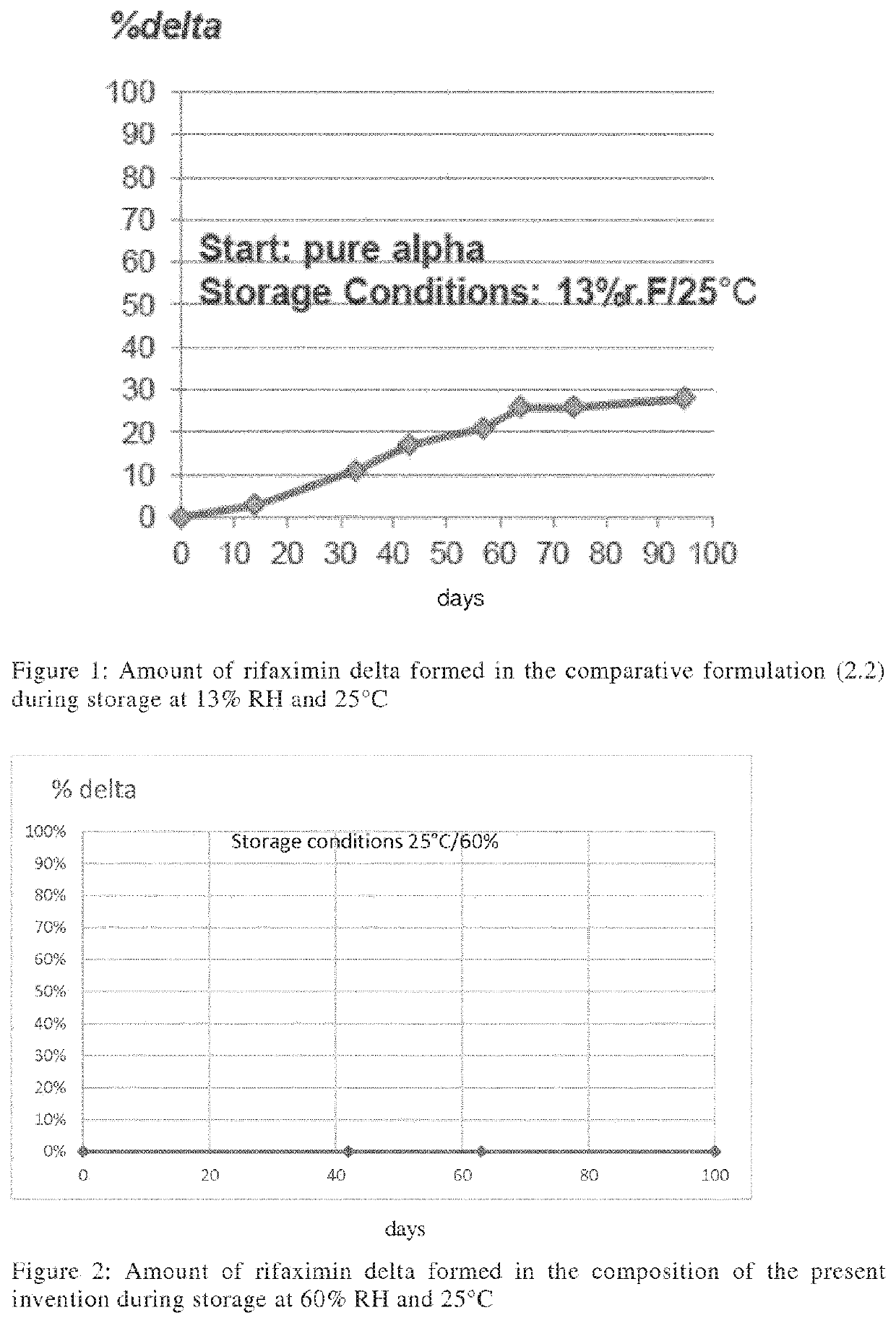
Storage stable composition comprising rifaximin alpha
The present invention relates to a pharmaceutical composition containing a stable polymorph of rifaximin and a wicking agent as well as a method of preparing the same.
Owner:SANDOZ AG
Oral dosage form comprising rifaximin in form beta
The present invention relates to an oral dosage form containing rifaximin in form beta, wherein the oral dosage form provides delayed release of the active pharmaceutical agent. Further, the invention relates to the preparation of an oral dosage form, preferably a tablet.
Owner:SANDOZ LTD
Novel parasite therapy
In alternative embodiments, provided are pharmaceutical compositions and methods for treating, ameliorating, reversing and / or preventing (acting as a prophylaxis) autism, e.g., regressive autism. In alternative embodiments, these pharmaceutical compositions and methods are dosaged and administered to children in need thereof. In alternative embodiments, pharmaceutical compositions and methods are dosaged, formulated and dosaged as solid, liquid or aerosol preparations or formulations. In alternative embodiments, pharmaceutical compositions comprise rifaximin as the sole antibiotic, or rixafimin and other antimicrobial or antibiotic agent, for example, vancomycin, metronidazole, tinidazole or a combination thereof.
Owner:BORODY THOMAS JULIUS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com