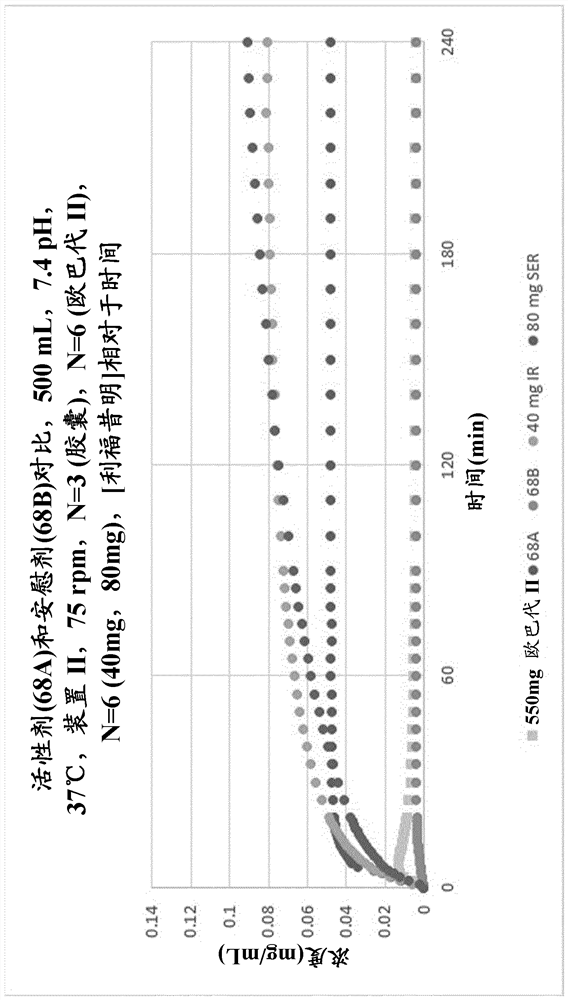
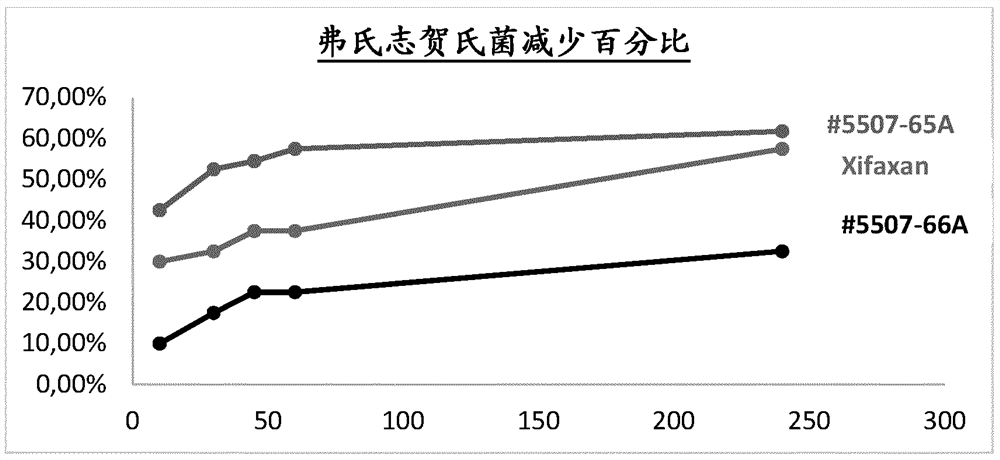
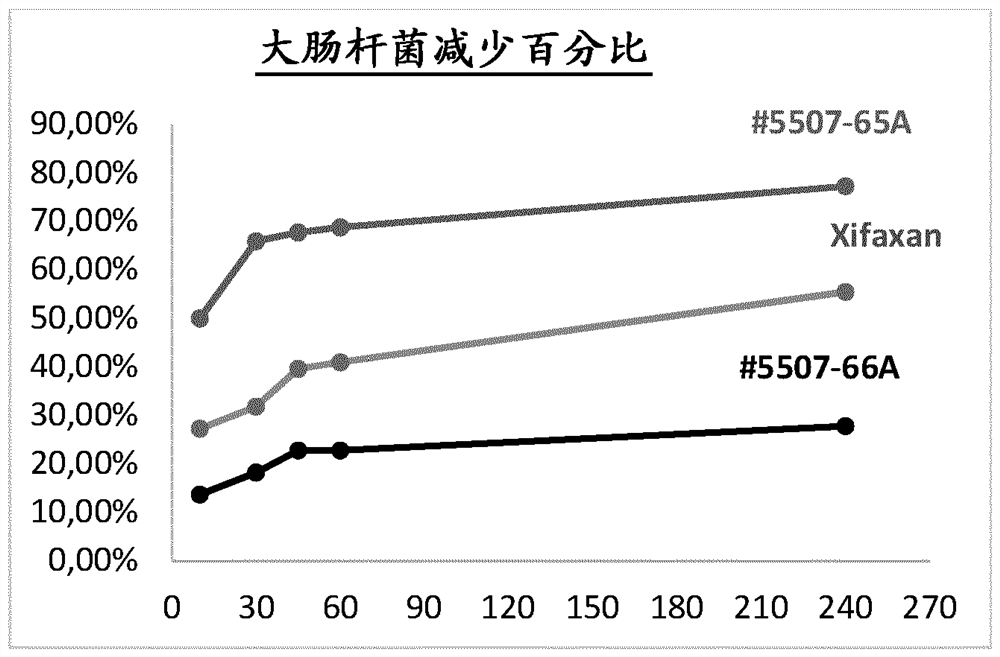
Rifaximin liquid preparation
A technology of rifaximin and excipients, applied in the field of rifaximin liquid preparation, can solve the problems of low systemic utilization rate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0078] Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs.
[0079] When ranges are used herein to describe, for example, amounts of a particular compound or ingredient, all combinations and subcombinations of ranges and specific embodiments therein are intended to be included. The use of the term "about" when referring to a number or numerical range means that the referenced number or numerical range is an approximation within experimental variability (or within statistical experimental error) and that therefore the number or value Ranges can vary. The term "comprising" (and related terms such as "containing" or "having" or "comprising") includes embodiments such as "consisting of" or "consisting essentially of" the described features An embodiment of any composition of matter, method, or process.
[0080] 1. Definition
[0081] "Rifaximin"...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com