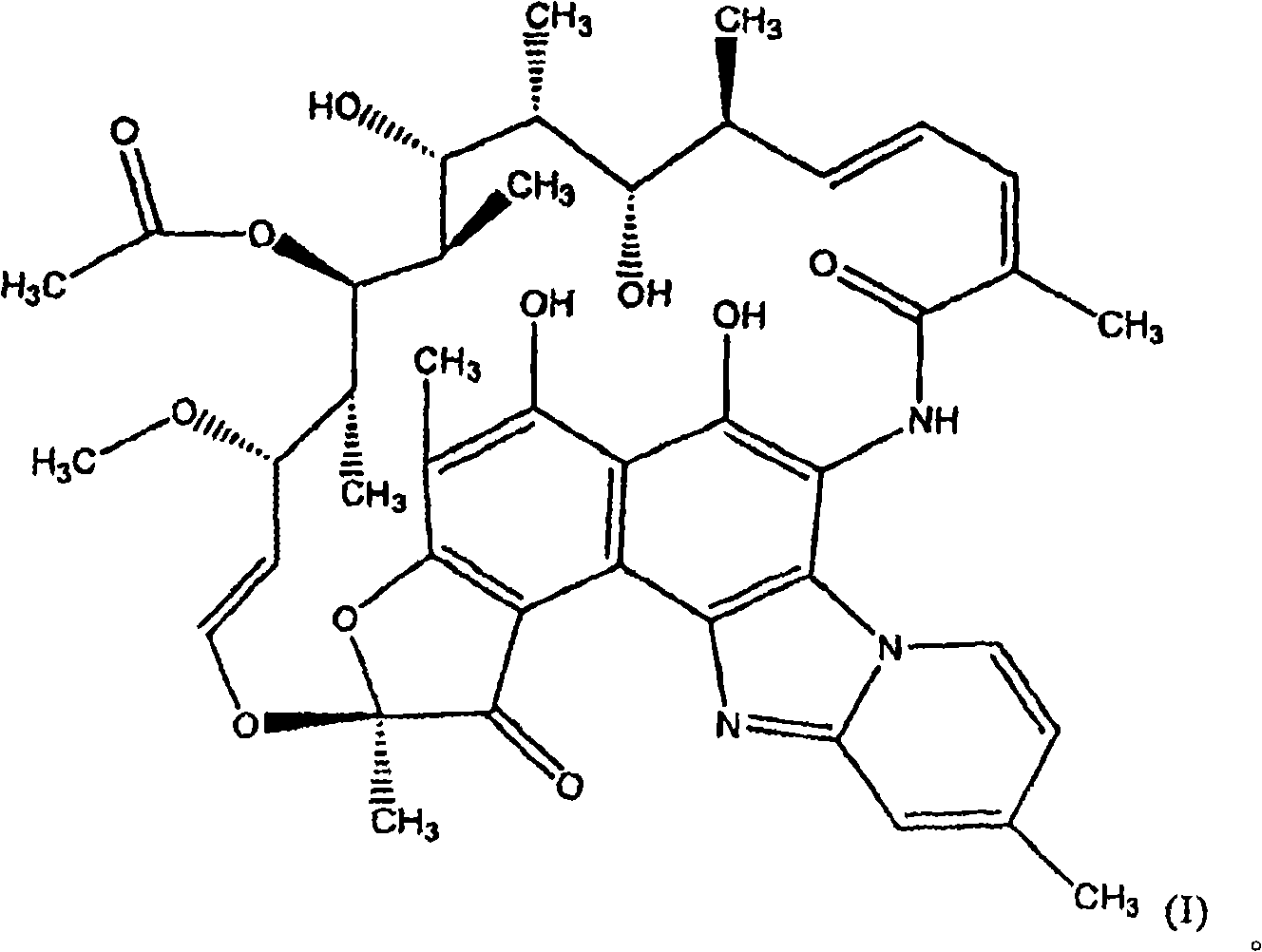
Rifaximin anti-rectal dysfunction preparation
A rifaximin and rectal technology, applied in the field of rifaximin anti-rectal dysfunction preparations, can solve problems such as shortening the healing time, increasing sensitivity, and finding no antibiotics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0186] Rifaximin tablets were dissolved in acetone until 200 mg of rifaximin was added to 30 g of ointment. Nitroglycerin is also added to the ointment.
Embodiment 2
[0188] By mixing 12.5 g of white petrolatum, lanolin, and distilled water containing 2% nitroglycerin (nitroglycerin ointment, USP 2%; E. Fougera & Co., Melville, N.Y.) with 37.5 g of white petrolatum at room temperature in a laboratory mixing vessel. Vaseline, USP (VASELINE; Chesebrough-Ponds USA Co., Greenwich, Conn.) and 100 mg of rifaximin were mixed to prepare an ointment.
Embodiment 3
[0190] 12.5 g of white petrolatum, lanolin, and distilled water (nitroglycerin ointment, USP 2%; E. Fougera & Co., Melville, N.Y.) containing 2% nitroglycerin with 20 g of 2.5% hydrogenated White petrolatum and light mineral oil for cortisone (hydrocortisone ointment, USP 2.5%; Clay-Park Labs, Inc., Bronx, N.Y.), 100 mg of rifaximin and 17.5 g of white petrolatum, USP (VASELINE; Chesebrough-Ponds USA Co., Greenwich, Conn.) mixing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com