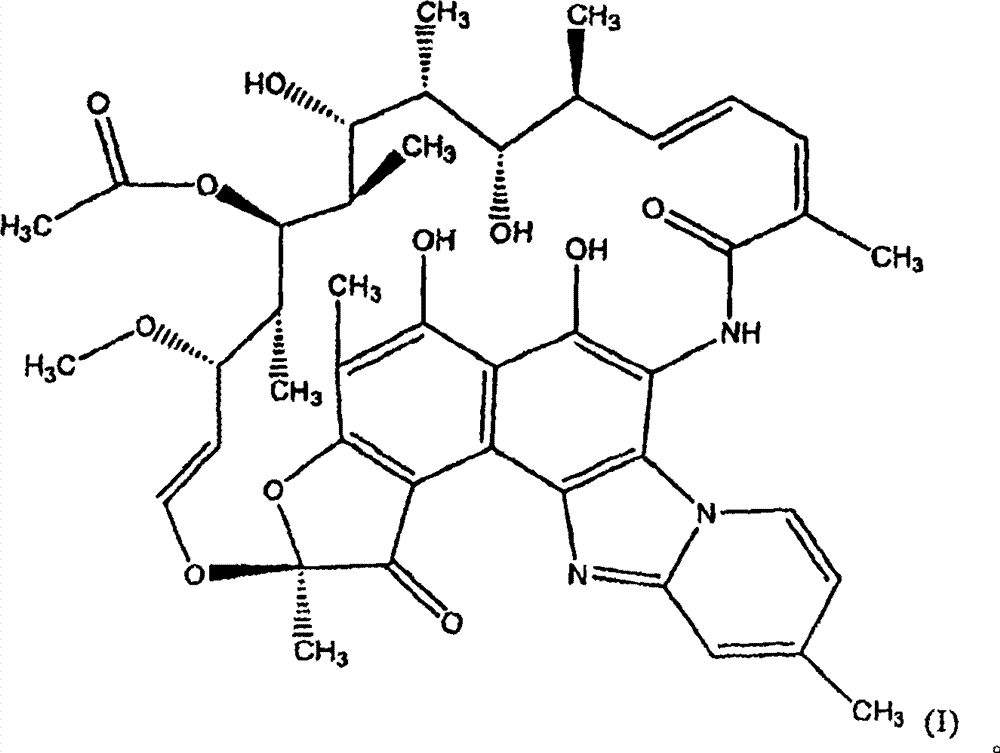
Rifaximin anti-rectal dysfunction preparation
A rifaximin and preparation technology, which is applied in the field of rifaximin anti-rectal dysfunction preparations, can solve the problems of no antibiotics, shortened healing time, sensitization and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0187] Rifaximin tablets were dissolved in acetone until 200 mg of rifaximin was added to 30 g of ointment. Nitroglycerin is also added to the ointment.
Embodiment 2
[0189] By mixing 12.5 g of white petrolatum, lanolin, and distilled water containing 2% nitroglycerin (nitroglycerin ointment, USP 2%; E. Fougera & Co., Melville, N.Y.) with 37.5 g of white petrolatum, USP, in a laboratory mixing vessel at room temperature. (VASELINE; Chesebrough-Ponds USA Co., Greenwich, Conn.) was mixed with 100 mg of rifaximin to prepare an ointment.
Embodiment 3
[0191] 12.5 g of white petrolatum containing 2% nitroglycerin, lanolin, and distilled water (nitroglycerin ointment, USP 2%; E. Fougera & Co., Melville, N.Y.) were mixed with 20 g of 2.5% hydrocortisone in a laboratory mixing vessel at room temperature. White petrolatum and light mineral oil (hydrocortisone ointment, USP 2.5%; Clay-Park Labs, Inc., Bronx, N.Y.), 100mg of rifaximin and 17.5g of white petrolatum, USP (VASELINE; Chesebrough- Ponds USA Co., Greenwich, Conn.) mixing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com