Novel veterinary uterus injectant as well as preparation method and application thereof
An injection, uterus technology, applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of neglect, endometrial damage, inability to take into account the repair of uterine epidermis, etc. To achieve the effect of accelerating cell repair, promoting proliferation, and speeding up the recovery of healthy physique
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
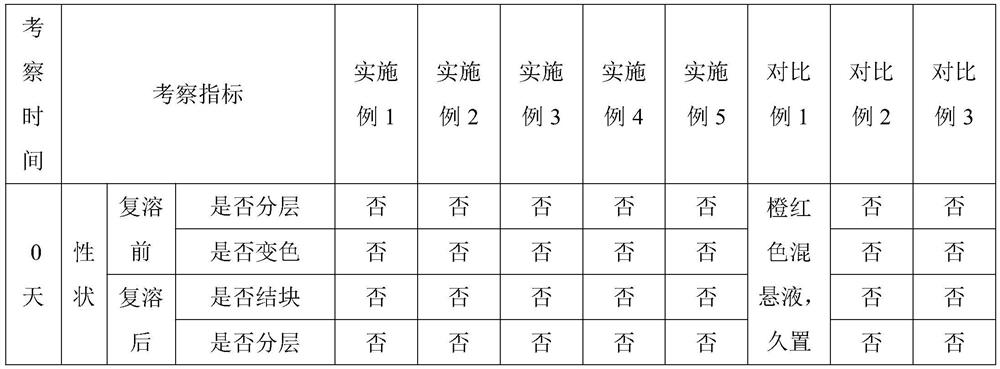
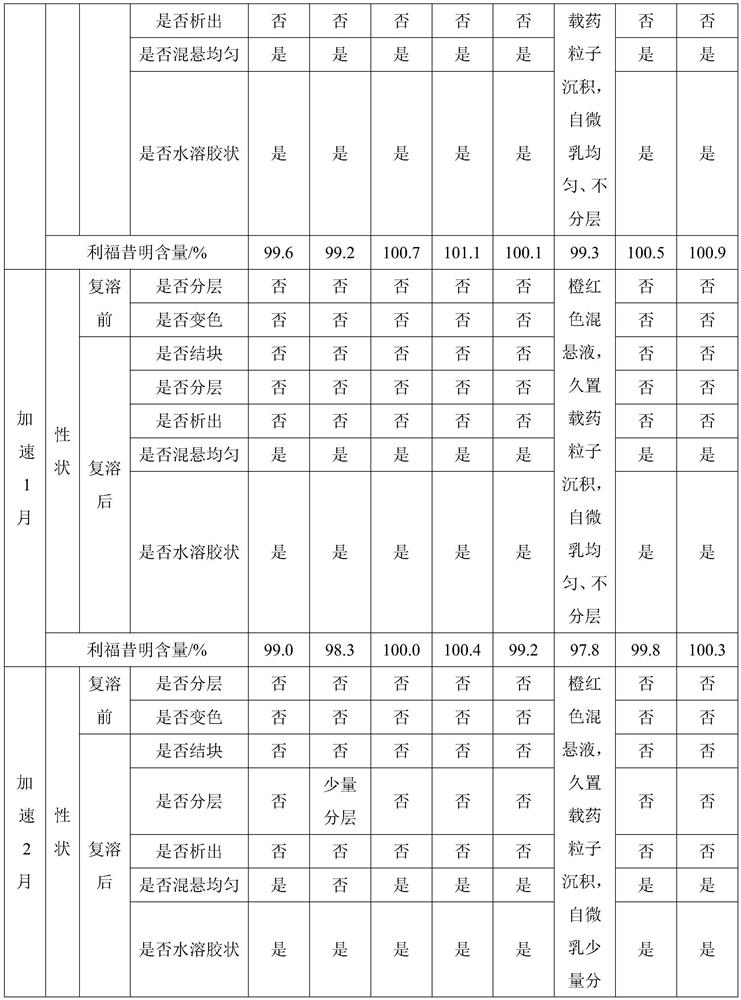
[0040] A new veterinary uterine injection, calculated by 100ml, consists of the following components: Rifaximin self-microemulsion 5g, adsorption carrier 1g, growth repair factor (oligopeptide-1 / blue copper peptide: collagen peptide: transparent Sodium hyaluronate=0.3:1:3) 4.3g, water-soluble silk fibroin 10g, GantrezAN 0.5g, the balance is water for injection;
[0041] Its preparation method comprises the following steps:
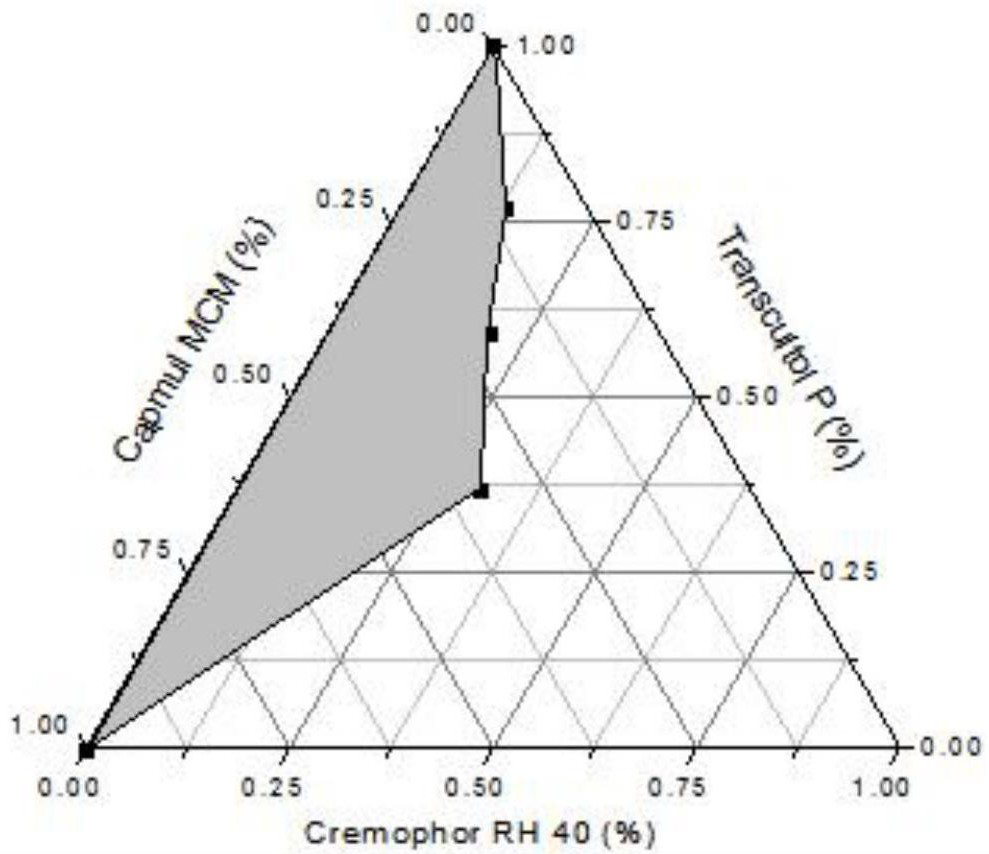
[0042] (1) Weigh 9.6g of polyoxyethylene ether hydrogenated castor oil (Cremophor RH 40) and 48g of diethylene glycol monoethyl ether (Transcutol P) and place them in a vortex mixer for vortex mixing, then add caprylic acid Add 38.4 g of capric monoglyceride (Capmul MCM) into the above mixture, vortex and mix to obtain a blank self-microemulsion, and dissolve 4 g of rifaximin into the blank self-microemulsion to obtain a rifaxime Mingzi microemulsion;
[0043] (2) Put calcium silicate (FLORITE PS-10) and hydrophilic fumed silica (AEROSIL 380) in a square...
Embodiment 2
[0049] Same as Example 1, the difference is that it does not contain adsorbents. In the process, rifaximin self-microemulsion and growth repair factor are directly added to the original step (5) to make rifaximin uterine injection. The specific steps are as follows:
[0050] A novel veterinary uterine injection, in 100ml, consists of the following components: rifaximin self-microemulsion 5g, growth repair factor (oligopeptide-1 / blue copper peptide: collagen peptide: sodium hyaluronate= 0.3:1:3) 4.3g, water-soluble silk fibroin 10g, GantrezAN 0.5g, the balance is water for injection;
[0051] Its preparation method comprises the following steps:
[0052] (1) Weigh 9.6g of polyoxyethylene ether hydrogenated castor oil (Cremophor RH 40) and 48g of diethylene glycol monoethyl ether (Transcutol P) and place them in a vortex mixer for vortex mixing, then add caprylic acid Add 38.4 g of capric monoglyceride (Capmul MCM) into the above mixture, vortex and mix to obtain a blank self-m...
Embodiment 3
[0056] Same as Example 1, the difference is that the adsorption carrier is a hydrophilic fumed silica, and the specific steps are as follows:
[0057] A new veterinary uterine injection, calculated by 100ml, consists of the following components: rifaximin self-microemulsion 5g, hydrophilic fumed silica 1g, growth repair factor (oligopeptide-1 / blue copper peptide: Collagen peptide: sodium hyaluronate = 0.3:1:3) 4.3g, water-soluble silk fibroin 10g, GantrezAN 0.5g, the balance is water for injection;
[0058] Its preparation method comprises the following steps:
[0059] (1) Weigh 9.6g of polyoxyethylene ether hydrogenated castor oil (Cremophor RH 40) and 48g of diethylene glycol monoethyl ether (Transcutol P) and place them in a vortex mixer for vortex mixing, then add caprylic acid Add 38.4 g of capric monoglyceride (Capmul MCM) into the above mixture, vortex and mix to obtain a blank self-microemulsion, and dissolve 4 g of rifaximin into the blank self-microemulsion to obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com