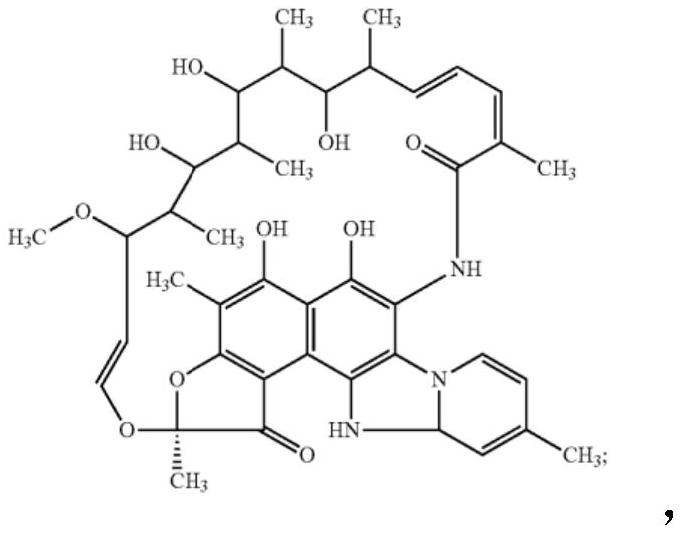
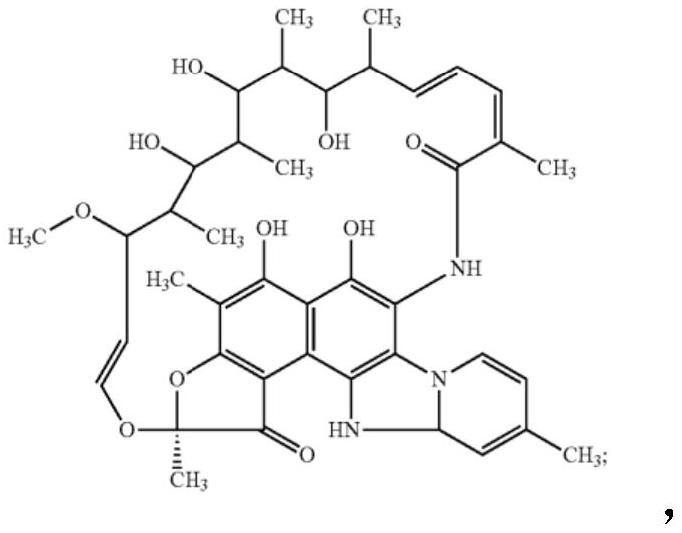
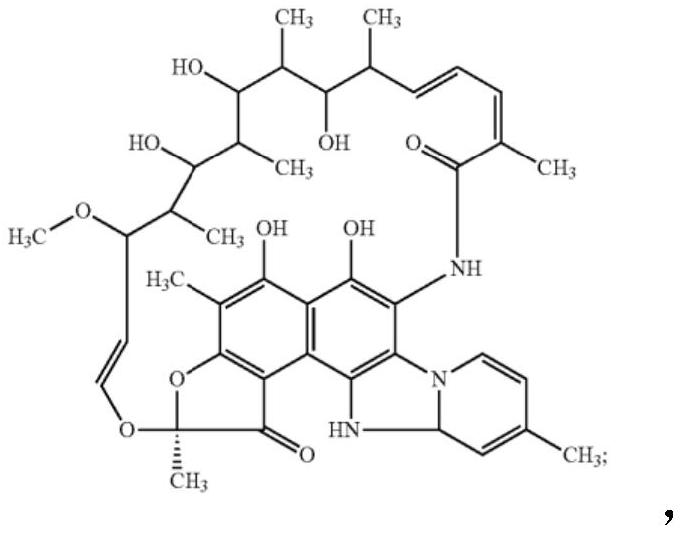
Compositions and methods for treating inflammatory bowel disease and fusobacteria-caused or related diseases and conditions
An inflammatory bowel disease, Fusobacterium technology, applied in the fields of medicine and gastroenterology, pharmacology and microbiology, can solve the problem of placebo effect differentiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0367] A 41-year-old female patient with a 12-year history of ulcerative colitis presented with 4-15 daily diarrheas and 2-3 nightly diarrheas. She had been treated with anti-inflammatory drugs including mesalamine, azathioprine and prednisone, and these achieved only transient responses. She also suffers from occasional bouts of urinary urgency and incontinence. Her initial colonoscopy revealed pancolitis.
[0368] She was started on secnidazole (400 mg three times daily) and rifampicin (increased from 150 mg twice daily to 300 mg twice daily after four weeks) and doxycycline (50 mg twice daily).
[0369] Over the next 6 to 8 weeks, the frequency of frequent movements slowly decreased to 3-6 times a day, bleeding was no longer seen and urgency had greatly improved. When the colonoscopy was repeated, she then continued on the same regimen for another six months. The aforementioned pancolitis is now greatly improved with almost complete healing of the mucosa. Biopsies showe...
example 2
[0371] A 42-year-old male patient with a 4-year history of Crohn's disease presented with a Crohn's disease activity index (CDAI) score of 550, 7-10 liquid stools per day, abdominal pain, inflammation, and deep ulcers microscopically. The patient was previously exposed to anti-TNF therapy, which was only transiently effective.
[0372] The patient was started on a combination of rifaximin (500 mg twice a day), tinidazole (500 mg twice a day) and nitazoxanide (500 mg twice a day). Each drug dose was increased by 500 mg after 2 weeks, with rifaximin slowly increasing to a final dose of 1.5 g twice a day (total of 3 g per day).
[0373] After 4 weeks, patients reported a significant reduction in liquid stools and abdominal pain. He observed a colonoscopy at 5 months, which showed excellent ulcer healing and significant improvement in inflammation. After another 4 months of treatment, the patient reported a more normal bowel movement frequency, roughly 3 soft stools a day, with ...
example 3
[0375] A 32-year-old male with long-standing ulcerative colitis (UC) showed on examination an extension of 45 cm from the anus because his initial treatment with immunomodulators failed to control his UC. A colonoscopy was performed on him and the inflammatory process was found to converge, starting at the anus and reaching approximately 40 cm. Collect cultures and biopsies. He has been treated with azathioprine and mesalamine plus steroids, but his Humira treatment has failed.
[0376] He started fosfomycin (1 g bid) in combination with doxycycline (50 mg bid) and metronidazole (400 mg bid) for 4 weeks.
[0377] His bloody stool frequency improved significantly after 2 weeks and his stools formed by 4 weeks of treatment. Bleeding stops after about a year of adhering to previous medication. At her 8-week colonoscopy, the microspots were still inflamed, but were generally marked as improved. With ongoing additional fecal microbiota transplant capsule treatment, the colon be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com