Rifaximin sustained-release preparation composition and method for preparing the same
A technology of rifaximin and sustained-release preparations, which is applied in the field of medicine, can solve problems such as poor patient compliance, and achieve the effect of good process quality controllability and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
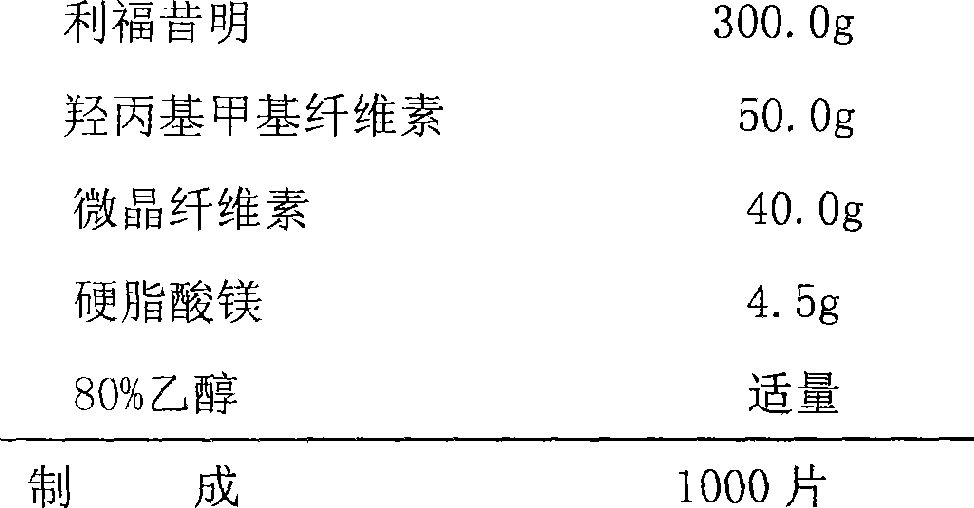
[0029] Sustained Release Tablets
[0030] prescription:
[0031]
[0032] Preparation Process:
[0033] Pass rifaximin, hydroxypropyl methylcellulose, microcrystalline cellulose, and magnesium stearate through an 80-mesh sieve respectively for later use, and weigh rifaximin, hydroxypropyl methylcellulose, microcrystalline Put the crystalline cellulose in a mixer and mix evenly, add an appropriate amount of 80% ethanol, granulate and granulate, dry at 45°C for 30 minutes, add the prescribed amount of magnesium stearate to the dry granules and mix evenly, pass through a 16-mesh sieve with a swing granulator Whole grains, measure the content of the granules, determine the weight range of the tablets, compress the tablets, dry, and pack after passing the inspection.
[0034] The rifaximin sustained-release tablet prepared by the above method meets the requirements of the relevant testing items stipulated in the "General Rules for Preparations" of "Chinese Pharmacopoeia 2005 E...
Embodiment 2
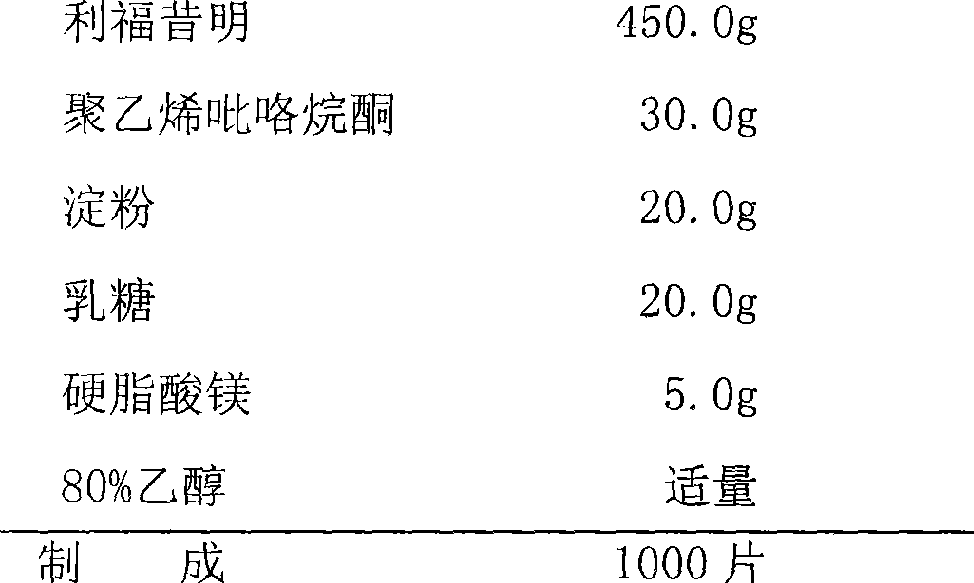
[0036] Sustained Release Tablets
[0037] Prescription: plain tablet
[0038]
[0039] Prescription: Sustained-release coating solution
[0040] 25 parts of hydroxypropyl methylcellulose
[0041] 10 parts ethyl cellulose
[0042] Propylene glycol 5 parts
[0043] Titanium dioxide 6 parts
[0044] 4 parts talcum powder
[0045] 2% ethyl cellulose ethanol solution appropriate amount
[0046] Preparation Process:
[0047] Pass rifaximin, polyvinylpyrrolidone, starch, lactose, and magnesium stearate through a 100-mesh sieve respectively, weigh rifaximin, polyvinylpyrrolidone, starch, and lactose according to the prescription amount, mix them in a mixer, and add Appropriate amount of 80% ethanol, granulate, dry at 45°C for 30 minutes, add the prescribed amount of magnesium stearate to the dry granules and mix well, pass through a 16-mesh sieve with a swinging granulator for granulation, measure the content of the granules, and determine the range of tablet weight , table...
Embodiment 3
[0050] Sustained Release Capsules
[0051] prescription:
[0052]
[0053] Preparation Process:
[0054]Pass rifaximin, hydroxypropyl methylcellulose, ethyl cellulose, lactose, and magnesium stearate through 80 mesh sieves respectively, and weigh rifaximin, hydroxypropyl methylcellulose, ethyl cellulose, Base cellulose, lactose, and magnesium stearate are placed in a mixer and mixed evenly, and an appropriate amount of 4% polyvinylpyrrolidone ethanol solution is used to make soft materials, and granulated in a granulator. The granules prepared above were dried at 60° C. for 1.5 h. Then pass through a swinging granulator, and use a 20-mesh sieve to sieve the granules. The content of the mixed granules is measured, and the capacity range of the ordinary hard capsule shell is determined to be filled, and the package is packaged after passing the inspection.
[0055] The rifaximin sustained-release capsules prepared by the above method meet the requirements of the relevant ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com