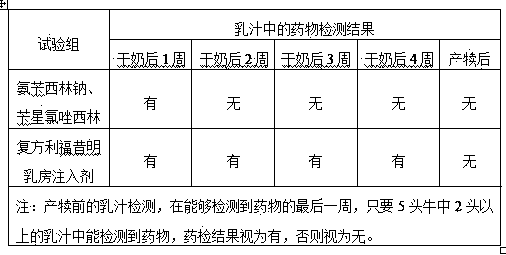
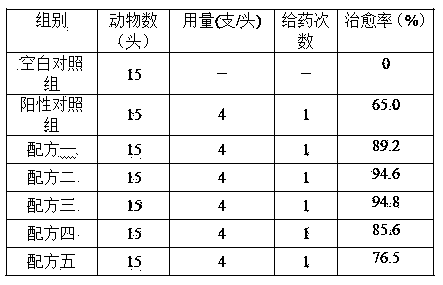
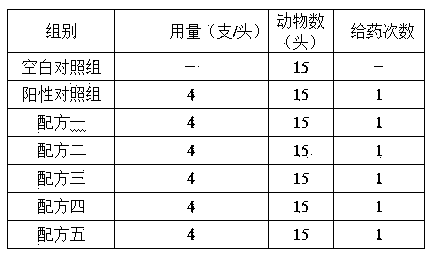
Rifaximin-containing preparation for preventing and treating dairy cow mastitis during dry period and method for preparing rifaximin-containing preparation
A technology of rifaximin during the dry period of dairy cows, which is applied in the field of udder injection drugs and its preparation, can solve the problems that the antibacterial effect is not as good as antibiotics, bacteria are prone to drug resistance, and antibiotic components are single, so as to enhance drug efficacy and reduce Drug residues in the body and the effect of reducing bacterial resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1) Put 82.9 parts (parts by weight, the same below) of pharmaceutical grade castor oil into a beaker and heat to 65 °C; then weigh 5 parts of thickener Poloxamer 407 and add it to the castor oil, stir until completely dissolved.
[0043] 2) Weigh 1.5 parts of viscosity modifier isopropyl myristate, heat to 65 °C, and dissolve 0.1 part of antioxidant BHT in it, and set aside.
[0044] 3) Weigh 0.5 parts of rifaximin and 5 parts of dandelion plant extract, add 5 parts of medicinal castor oil, and fully ultrasonically mix to make them completely dispersed.
[0045] 4) Pour 2) viscosity modifier into 1) step pharmaceutical grade castor oil while it is hot, stir until completely dissolved, let cool to room temperature; then pour 3) step raw material into 1), and mix well for 1 hour , to make it completely dispersed, and finally use a high-speed shear emulsifier to stir for 30 minutes under the condition of 50Hz, let it cool and pack it. A preparation containing 0.5% rifaxim...
Embodiment 2
[0047] 1) Put 82.4 parts of pharmaceutical grade castor oil into a beaker and heat to 65 °C; then weigh 5 parts of thickener poloxamer 407 and add castor oil and stir until completely dissolved.
[0048] 2) Weigh 1.5 parts of viscosity regulator isopropyl myristate, heat to 65 °C, and dissolve 0.1 part of antioxidant BHT in it, and set aside.
[0049] 3) Weigh 0.5 parts of rifaximin and 5.5 parts of dandelion plant extract, add 5 parts of medicinal castor oil, and fully ultrasonically mix to make them completely dispersed.
[0050] Pour the viscosity modifier in step 2) into the pharmaceutical grade castor oil in step 1) while it is hot, stir until it is completely dissolved, and let it cool to room temperature; then pour the raw material drug in step 3) into step 1), mix well for 1 hour, Make it completely dispersed, and finally use a high-speed shear emulsifier to stir for 30 minutes under the condition of 50Hz, let it cool and pack it. A preparation containing 0.5% rifaxim...
Embodiment 3
[0052] 1) Put 81.9 parts of pharmaceutical grade castor oil into a beaker and heat to 65 °C; then weigh 5 parts of thickener Poloxamer 407 and add it to the castor oil, stir until completely dissolved.
[0053] 2) Weigh 1.5 parts of viscosity regulator isopropyl myristate, heat to 65 °C, and dissolve 0.1 part of antioxidant BHT in it, and set aside.
[0054] 3) Weigh 1 part of rifaximin and 5.5 parts of dandelion plant extract, add 5 parts of medicinal castor oil, and fully ultrasonically mix to make it completely dispersed.
[0055] 4) Pour the viscosity modifier in step 2) into the pharmaceutical grade castor oil in step 1) while it is hot, stir until it is completely dissolved, let it cool to room temperature; then pour the raw material drug in step 3) into 1), and mix well hour, make it completely dispersed, and finally use a high-speed shear emulsifier to stir for 30 min under the condition of 50Hz, let it cool and pack it. A preparation containing 1% rifaximin and 5.5% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com