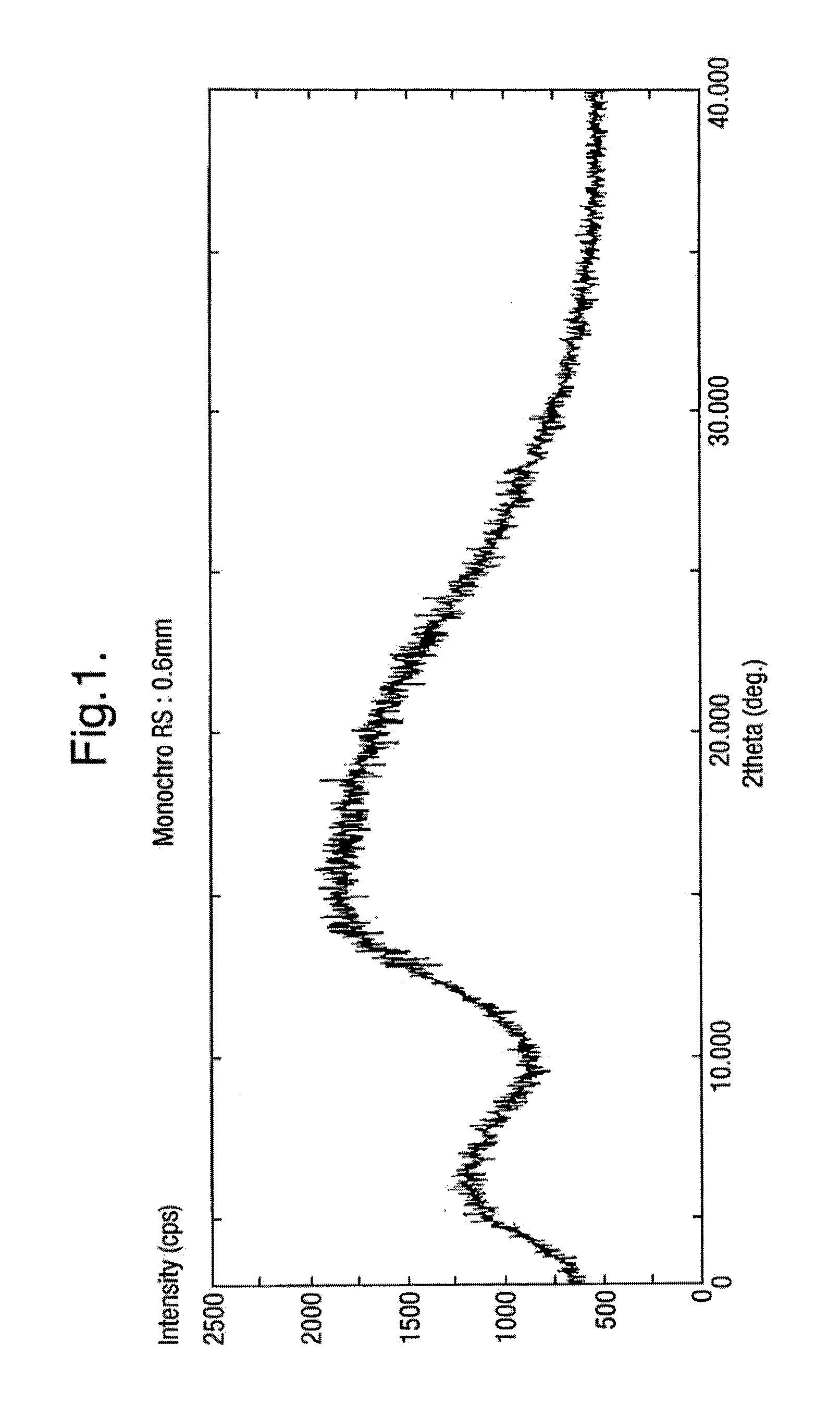
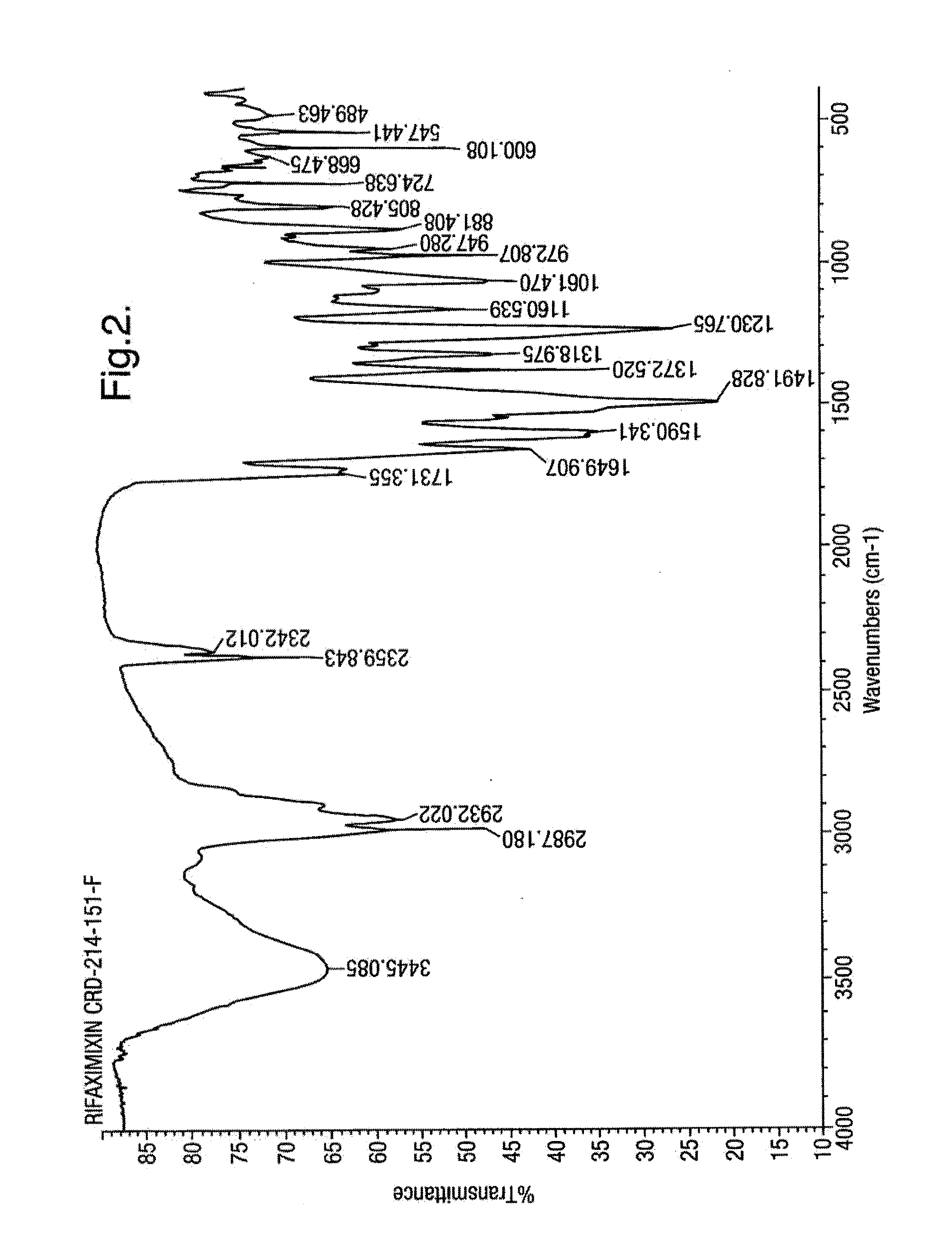
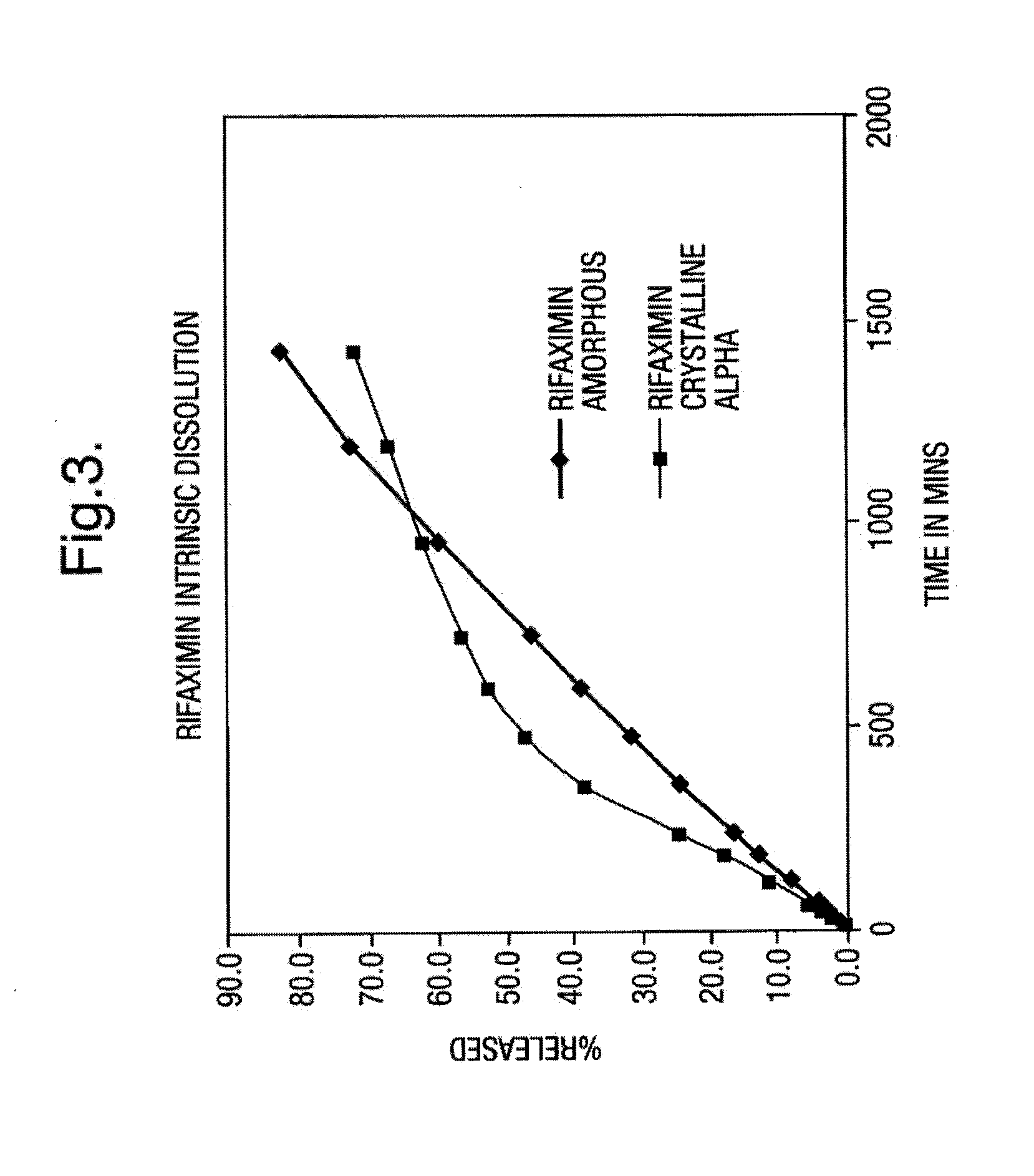
Rifaximin
a technology of rifaximin and amorphous form, which is applied in the field of rifaximin, can solve the problem that none of the above patents discloses a wholly amorphous form of rifaximin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0061]Rifamycin S 100 g (0.143 moles), dichloromethane 300 ml and 2-amino-4-picoline, 46.4 g (0.434 moles) were mixed at room temperature under argon atmosphere. Iodine 19 g (0.074 moles) dissolved in dichloromethane 700 ml, was added dropwise in 30-45 minutes at room temperature. Reaction mixture was then stirred at room temperature for 15-18 hours. L(−) Ascorbic acid 20 g (0.113 moles) dissolved in 100 ml water was added. The mixture was stirred for 30-45 minutes at room temperature and then cooled to 10 to 15° C. The pH of the reaction mixture was adjusted to 2 using 12.5% dil. HCl solution. The mass was stirred for 10 to 15 minutes, organic layer was separated and washed at first with demineralized water then with 10% sodium thiosulfate and finally with water till neutral pH was obtained. The organic layer was charcolized, filtered through hyflo, dried over sodium sulfate and concentrated under vacuum below 50° C. The product was stripped out with n-heptane and crude material th...
example 2
[0062]Amorphous rifaximin (100 g) was dissolved in acetic acid (200 ml) at 50° C., stirred for 30-45 minutes and demineralized water (200 ml) was added dropwise at 50° C. in 30-45 minutes. Stirring was continued at 50° C. for 30-45 minutes, cooled gradually to room temperature and stirred for 2 hours. The solid obtained was filtered and washed at first with acetic acid-water 1:1 mixture then with 10% acetic acid-water mixture and finally washed with water. The solid obtained was dried at 100-110° C. for 12-15 hours to get 62-65 g of rifaximin-γ-form.
example 3
[0063]Amorphous rifaximin (100 g) was dissolved in formic acid (200 ml) at 50° C., stirred for 30-45 minutes and demineralized water (200 ml) was added dropwise at 50° C. in 30-45 minutes. Stirring was continued at 50° C. for 30-45 minutes, cooled gradually to room temperature and stirred for 2 hours. The solid obtained was filtered and washed at first with formic acid-water 1:1 mixture then with 10% formic acid-water mixture and finally washed with water. The solid obtained was dissolved in Isopropyl alcohol (310 ml) at 50° C. and stirred at 50° C. for 30 minutes. Demineralized water (310 ml) was added dropwise at 50° C. in 30-45 minutes and stirring was continued at the same temperature for 30-45 minutes. The mixture was cooled gradually to room temperature and stirred for 2 hours. The solid obtained was filtered, washed with Isopropyl alcohol-water 1:1 mixture and then with demineralized water, dried at 80-90° C. for 10-15 hours to get 40-45 g of rifaximin-β-form.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| 2θ | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com