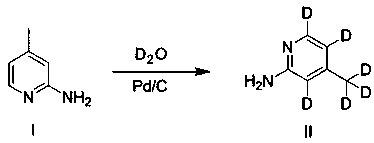
Synthesis process of rifaximin-D6
A technology of rifaximin and synthesis process, which is applied in the field of synthesis technology of rifaximin-D6, can solve the problem that 4-methyl-2-aminopyridine-D6 is not commercially available, the synthesis route is complicated, and there is no synthesis significance, etc. problem, to achieve the effect of easy operation, easy availability of raw materials, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The synthetic technique of embodiment 1 rifaximin-D6
[0032] first step:
[0033]
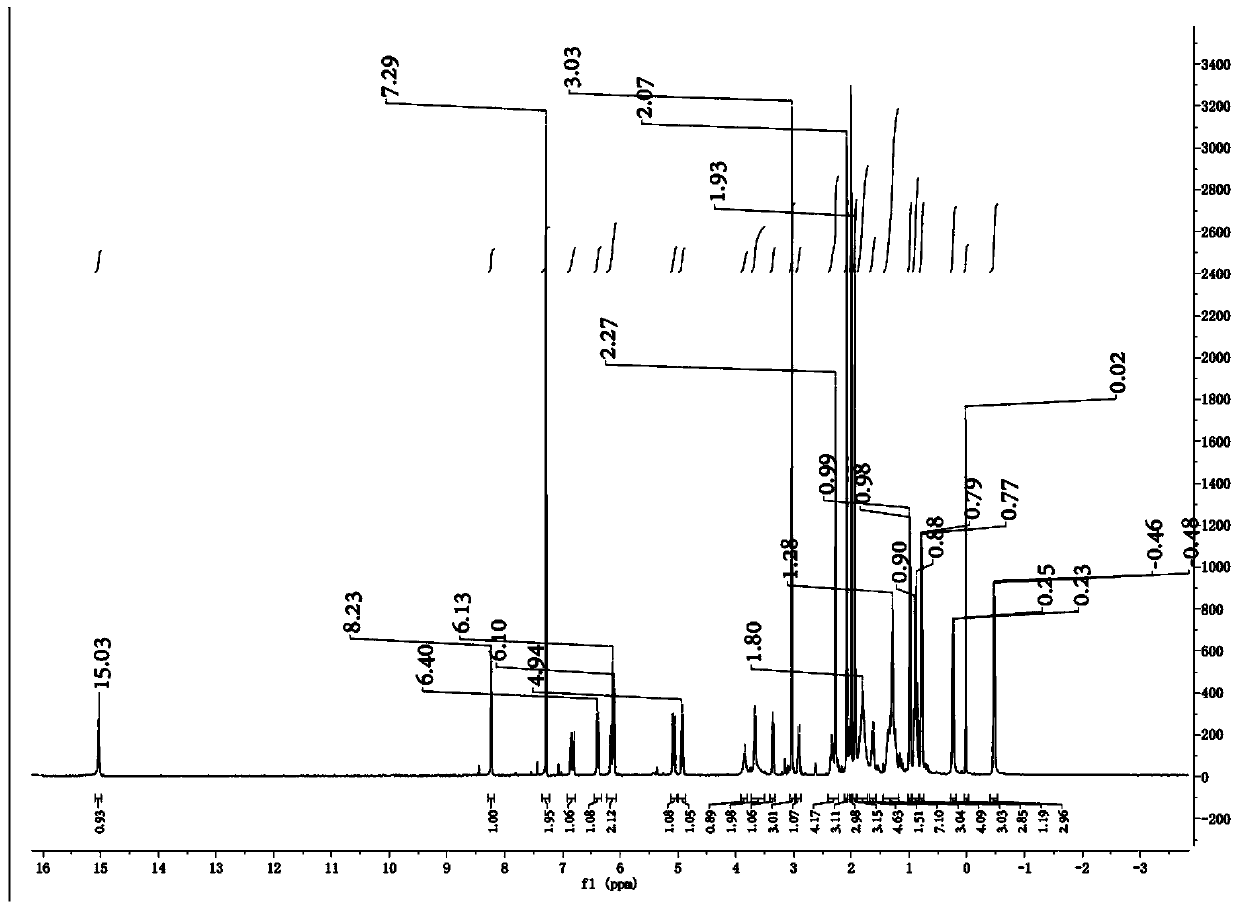
[0034] Add 6.5 g of compound I 2-amino-4-picoline (60.1 mmol) into the autoclave, add commercially available wet Pd / C 980 mg (Pd loading is 5 wt%), D 2 O (heavy water) 60ml (3.327mol), under nitrogen protection, react at 180°C for 16 hours, cool to room temperature, extract with dichloromethane (30mL*3), wash the organic phase once with water, once with saturated brine, and dry. Spin to dryness and column chromatography to obtain 4.610 g of pure compound II 4-methyl-2-aminopyridine-D6.
[0035] The second step: 1.22 grams of Rifamycin-S (rifamycin S) (1.75mmol), 610 mg of compound II (5.34mmol) were dissolved in 30 milliliters of DCM (dichloromethane), nitrogen protection, stirring at room temperature, 236.2 Dissolve milligrams of elemental iodine in 20 ml of DCM, add it dropwise to the system for about 45 minutes, then react overnight at room temperature for about 18 hours, dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com