Use of tigecycline, alone, or in combination with rifampin to treat osteomyelitis and/or septic arthritis
A technology of tigecycline and rifampicin, applied in bone marrow, treatment of bacterial infection of bone, joint and synovial fluid, infection of antibiotic-resistant bacteria, can solve problems such as limitations of oral antibacterial agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
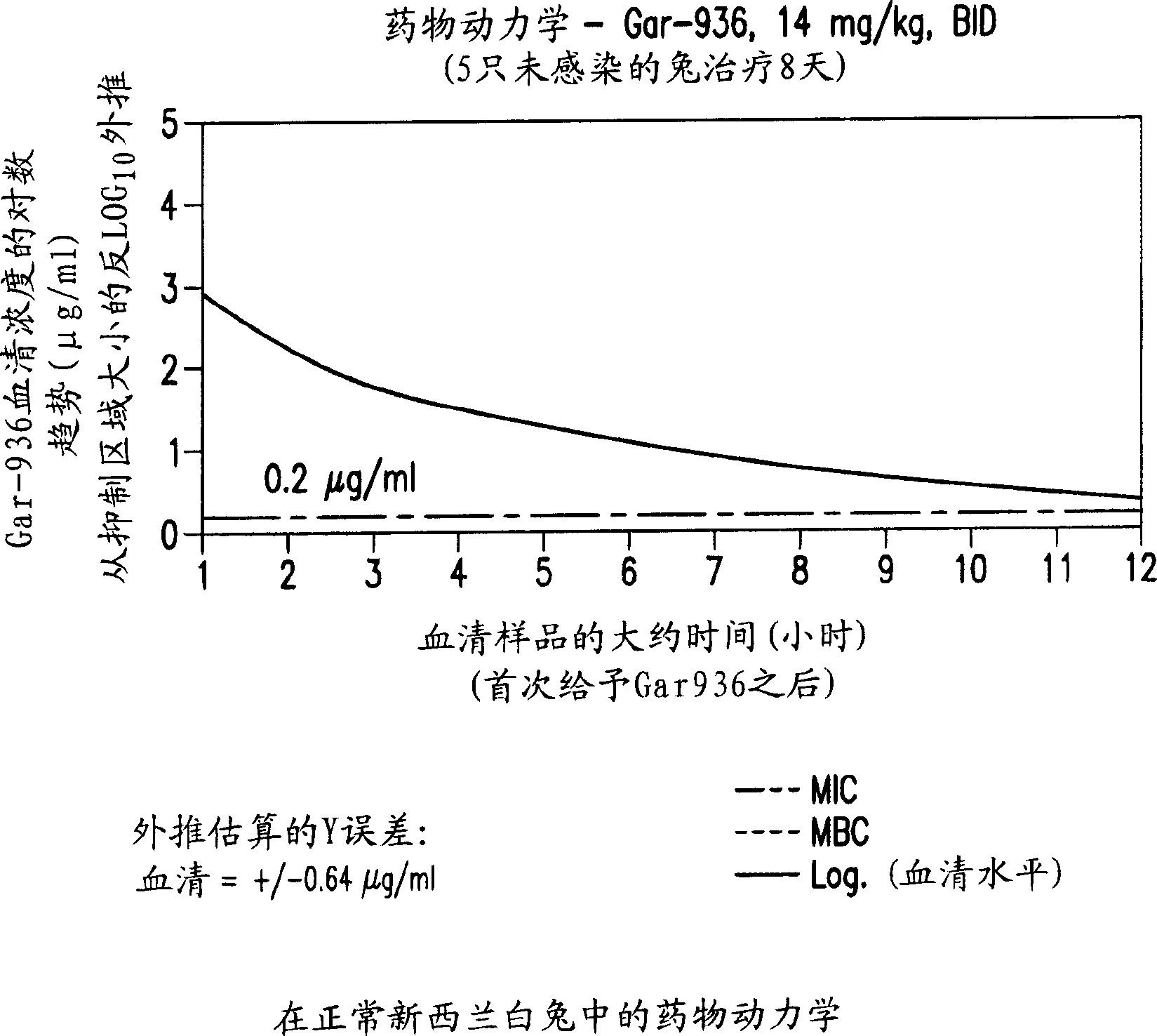
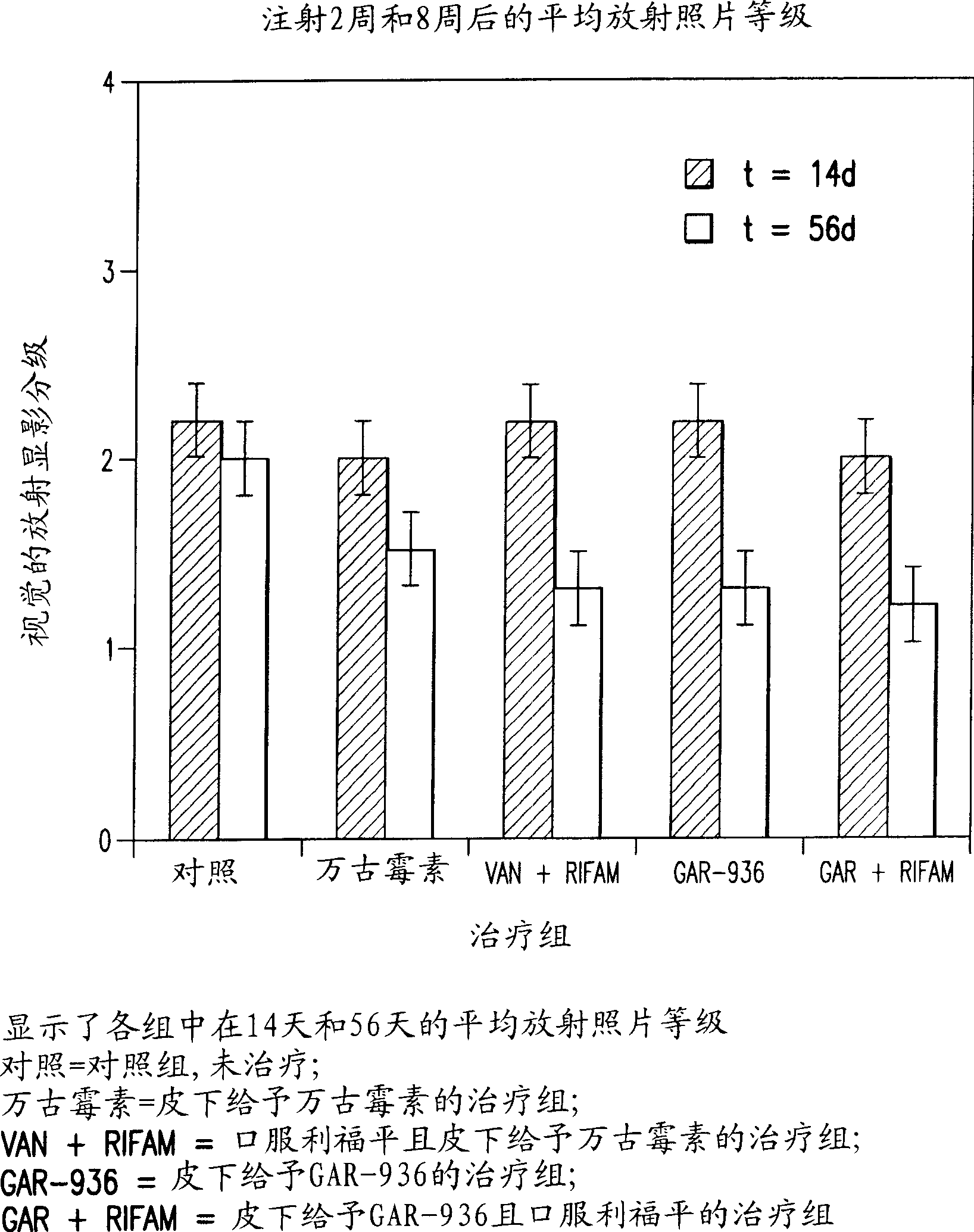
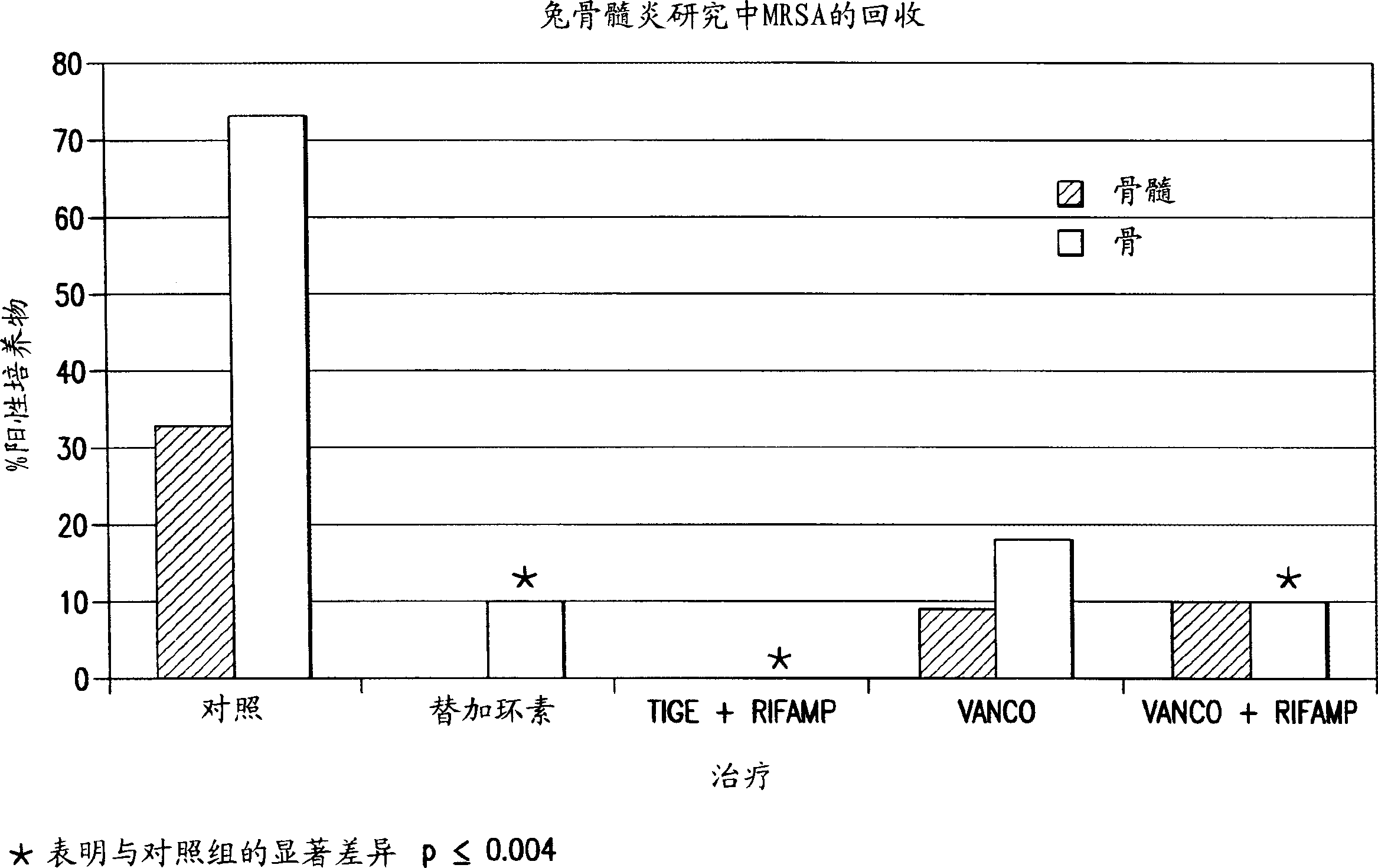
[0091] Example 1: Treatment of Osteomyelitis Using Tigecycline in Rabbits
[0092] This example demonstrates the use of tigecycline and the combination of tigecycline and rifampicin for the treatment of osteomyelitis in rabbits. Comparative studies using vancomycin and vancomycin plus rifampicin have also been performed. The data demonstrate improved antimicrobial efficacy with tigecycline versus vancomycin and with tigecycline plus rifampicin versus vancomycin plus rifampicin. Furthermore, within the test group, tigecycline plus rifampicin provided complete protection against methicillin-resistant S. aureus.
[0093] Standard curve generated by diffusion bioassay
[0094] Tigecycline (Wyeth-Ayerst Research, Pearl River, New York), vancomycin (Abbott Laboratories, Chicago, Illinois) and rifampin were produced using normal NZW rabbit serum (Fisher Sciontific) and normal, uninfected rabbit tibiae. A standard curve was obtained from Ping (Merrell Pharmaceuticals Inc. Kansas, Mis...
Embodiment 2
[0142] Example 2: Distribution of tigecycline in human tissues after a single intravenous administration of 100 mg.
[0143] This example demonstrates the penetration of selected tissues in human subjects following a single intravenous administration of tigecycline. The data demonstrate a rapid period of distribution with an extended half-life and a high volume of distribution at steady state. Penetration of bone, synovial fluid, lung, gallbladder and colon was further demonstrated in human subjects. Penetration improves the treatment of bone and joint infections.
[0144] Pharmacokinetic studies of intravenous tigecycline in humans have shown a rapid phase of distribution, a prolonged half-life (40-60 hours) and a high volume of distribution at steady state. Animal studies using radiolabeled tigecycline indicate that this rapid distribution phase and high volume of distribution at steady state represent penetration of tigecycline into tissues including lung and bone. Carbo...
Embodiment 3
[0177] Example 3: Tissue Distribution in Rats Treated with Tigecycline
[0178] This study aimed to quantify the organization of [ 14 C]-radioactivity produced by tigecycline in a single 30-minute intravenous infusion of 3 mg / kg in male Sprague-Dawley and Long-Evans rats [ 14 C] - Whole-mount autoradiography using fluorescence imaging after tigecycline.
[0179] Materials and methods
[0180] Tigecycline was provided by the Analytical Department, Wyeth-Ayerst Research, Montreal, Canada. [ 14 C]-Tigecycline was supplied by Amersham (Boston, MA). main body[ 14 C]- The radiochemical purity and specific radioactivity of tigecycline are 98% and 93.6 microCi / mg, respectively.
[0181] Use sterile water to prepare solutions for intravenous administration. The liquid scintillation mixture used to count radioactivity in plasma and urine was Ultima Gold (Packard Instruments Co., Meriden, CT).
[0182] A Model 3078 Tri-Carb Sample Oxidizer equipped with an Oximate-80 Robotic Auto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com