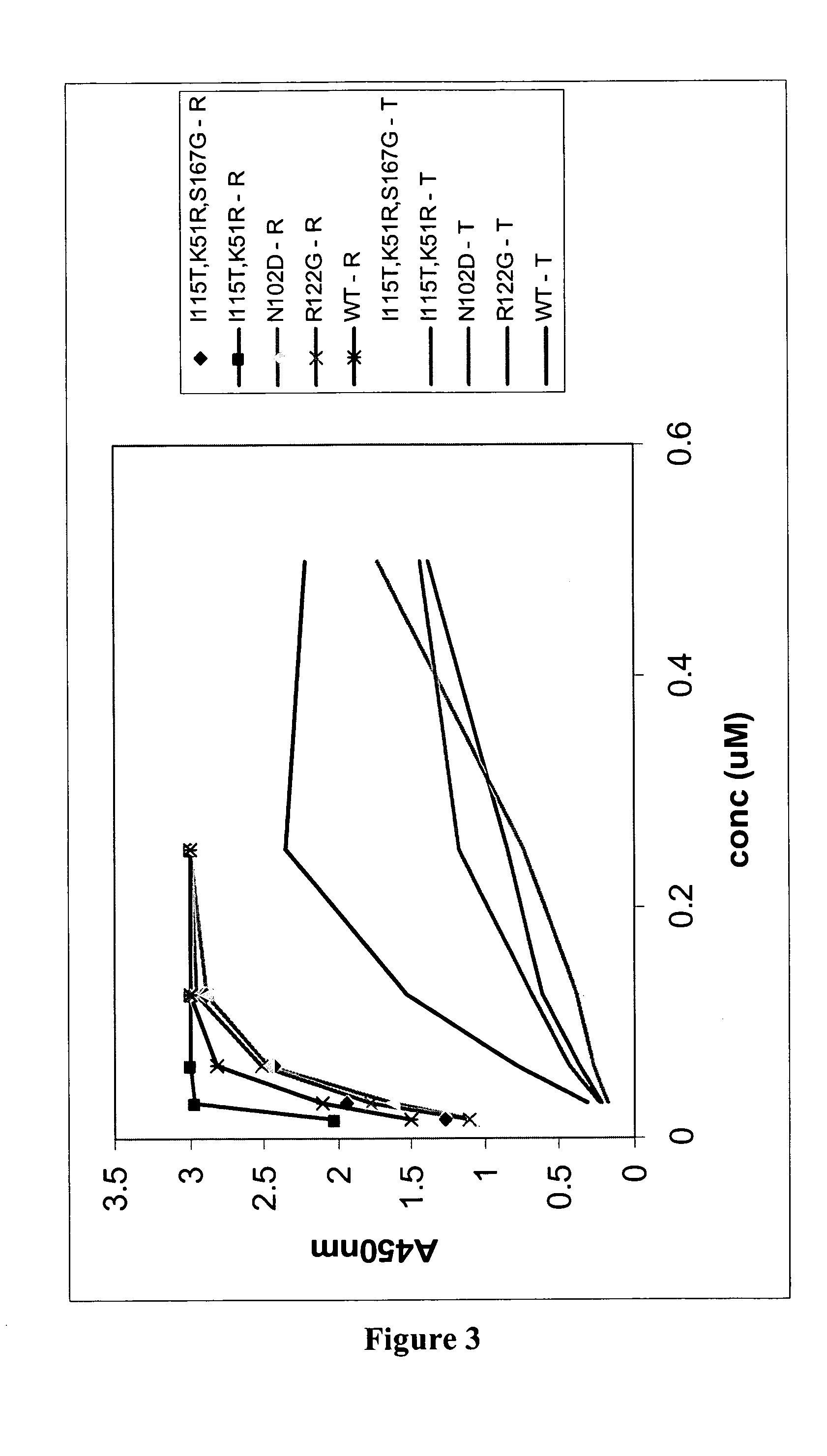
Patents
Literature
233 results about "Tuberculous osteomyelitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Osteomyelitis is a secondary complication in 1–3% of patients with pulmonary tuberculosis. In this case, the bacteria, in general, spread to the bone through the circulatory system, first infecting the synovium (due to its higher oxygen concentration) before spreading to the adjacent bone.
Treatment and diagnosis of macrophage mediated disease
The invention relates to a method of treating or monitoring / diagnosing a disease state mediated by activated macrophages. The method comprises the step of administering to a patient suffering from a macrophage mediated disease state an effective amount of a composition comprising a conjugate or complex of the general formulaAb−Xwhere the group Ab comprises a ligand capable of binding to activated macrophages, and when the conjugate is being used for treatment of the disease state, the group X comprises an immunogen, a cytotoxin, or a compound capable of altering macrophage function, and when the conjugate is being used for monitoring / diagnosing the disease state, X comprises an imaging agent. The method is useful for treating a patient suffering from a disease selected from the group consisting of rheumatoid arthritis, ulcerative colitis, Crohn's disease, inflammation, infections, osteomyelitis, atherosclerosis, organ transplant rejection, pulmonary fibrosis, sarcoidosis, and systemic sclerosis.
Owner:LOW PHILIP S +1
Diagnosis of macrophage mediated disease
InactiveUS20070231266A1Reduce inflammationReduce retentionAntibacterial agentsNervous disorderUlcerative colitisImaging agent
The invention relates to a method of treating or monitoring / diagnosing a disease state mediated by activated macrophages. The method comprises the step of administering to a patient suffering from a macrophage mediated disease state an effective amount of a composition comprising a conjugate or complex of the general formula Ab-X where the group Ab comprises a ligand capable of binding to activated macrophages, and when the conjugate is being used for treatment of the disease state, the group X comprises an immunogen, a cytotoxin, or a compound capable of altering macrophage function, and when the conjugate is being used for monitoring / diagnosing the disease state, X comprises an imaging agent. The method is useful for treating a patient suffering from a disease selected from the group consisting of rheumatoid arthritis, ulcerative colitis, Crohn's disease, inflammation, infections, osteomyelitis, atherosclerosis, organ transplant rejection, pulmonary fibrosis, sarcoidosis, and systemic sclerosis.
Owner:LOW PHILIP S +1
Bone implants for the treatment of infection
ActiveUS8221396B2Improve adhesionPrevent leechingBone implantPharmaceutical delivery mechanismDiseaseControl release
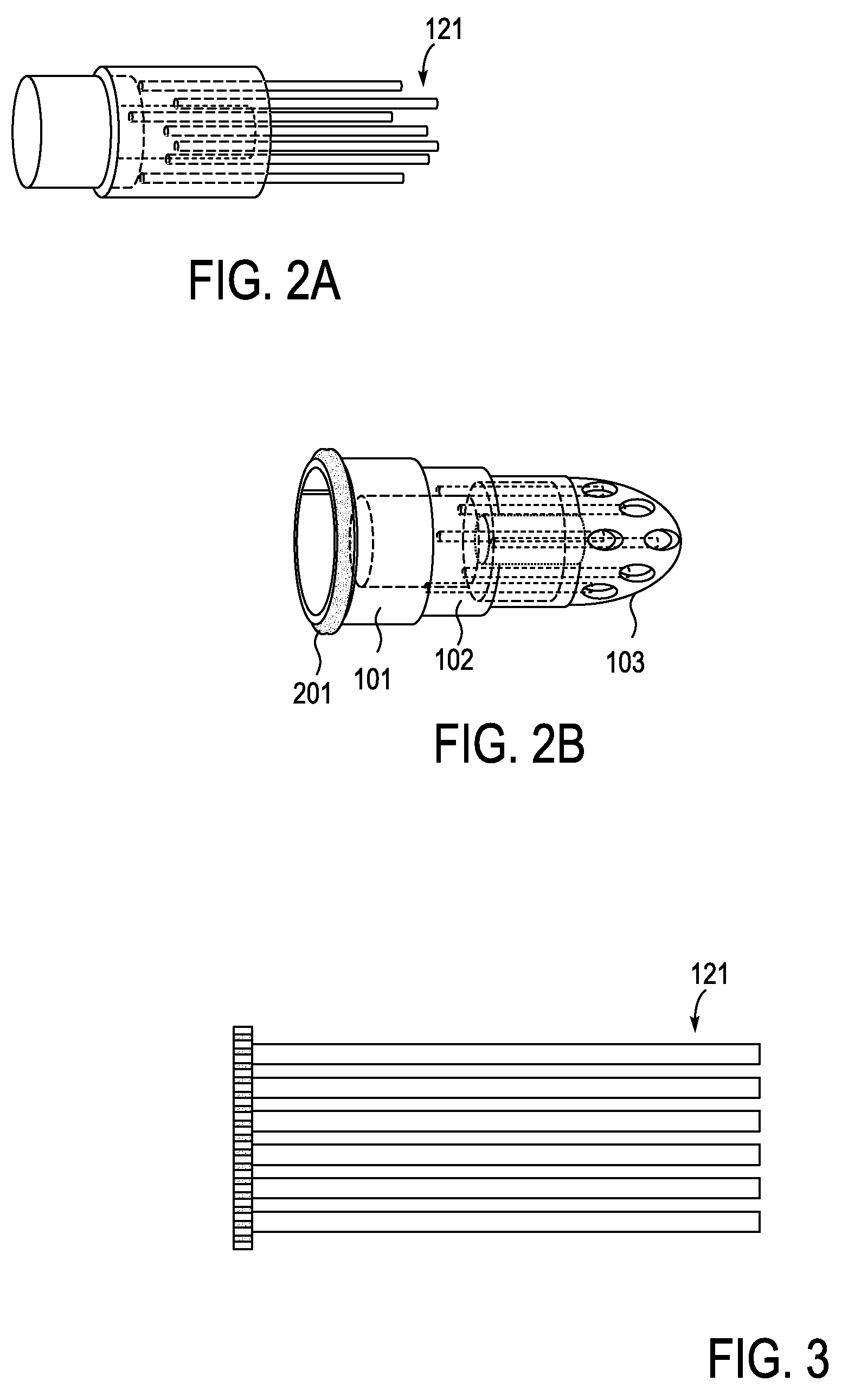
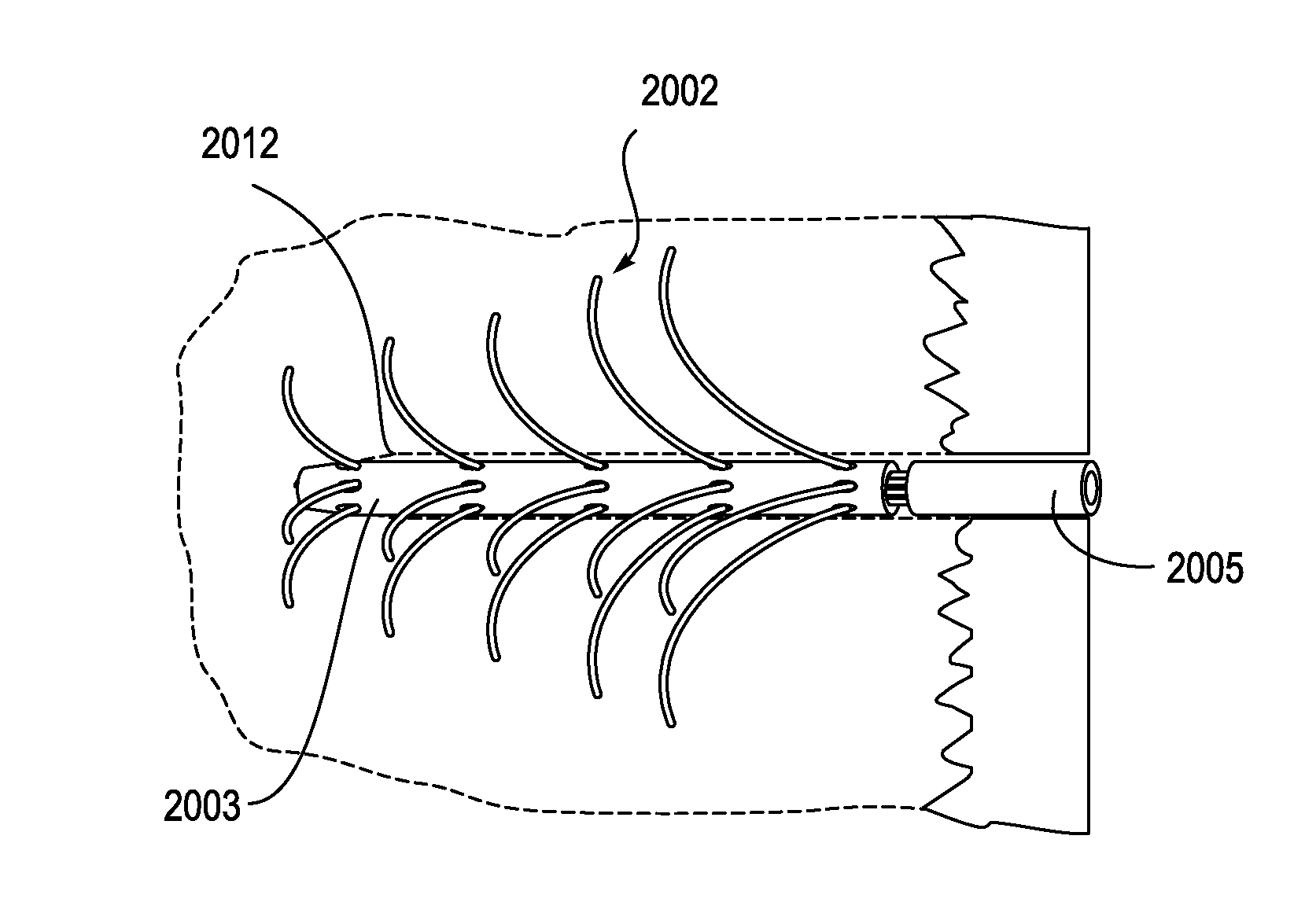
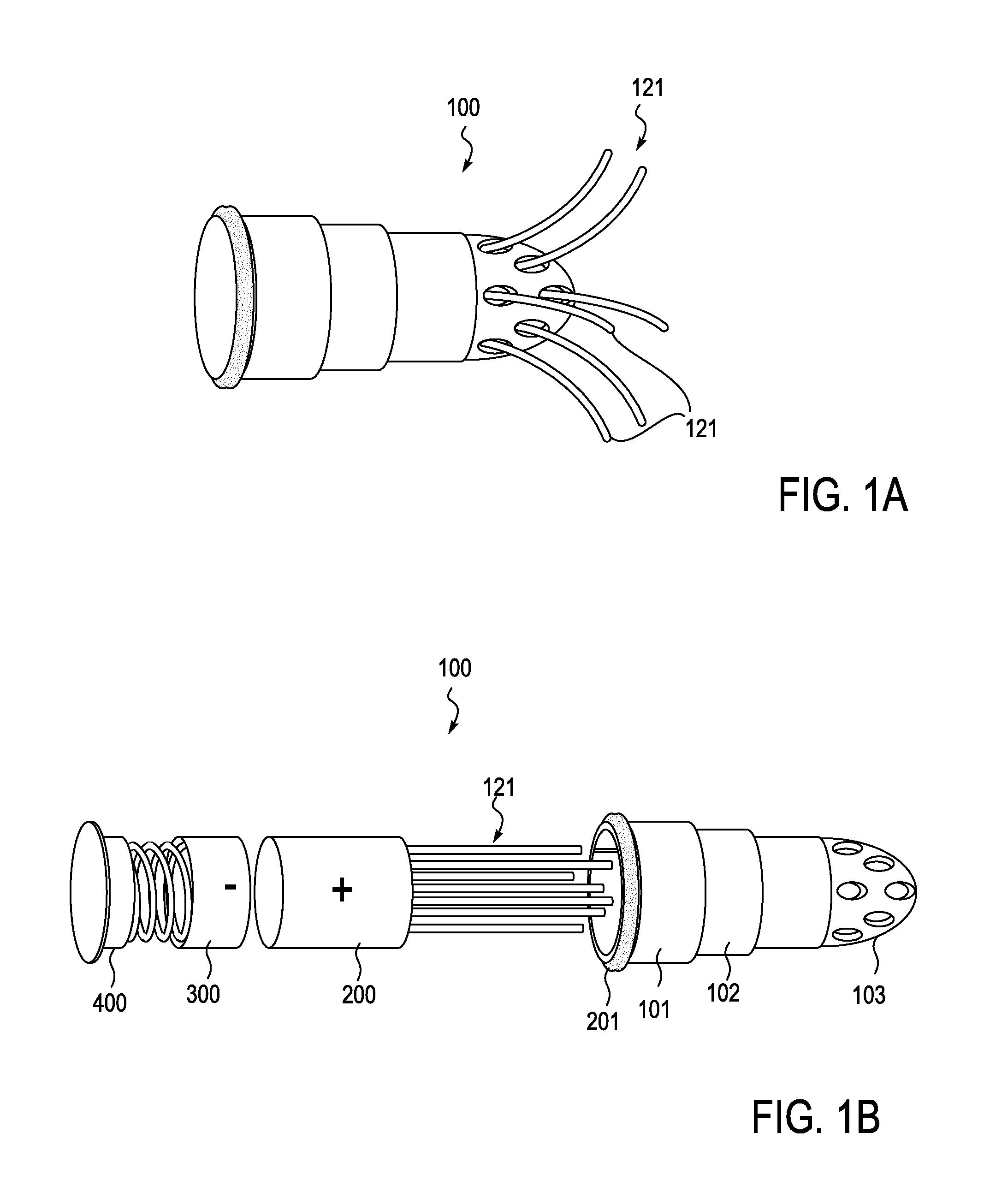
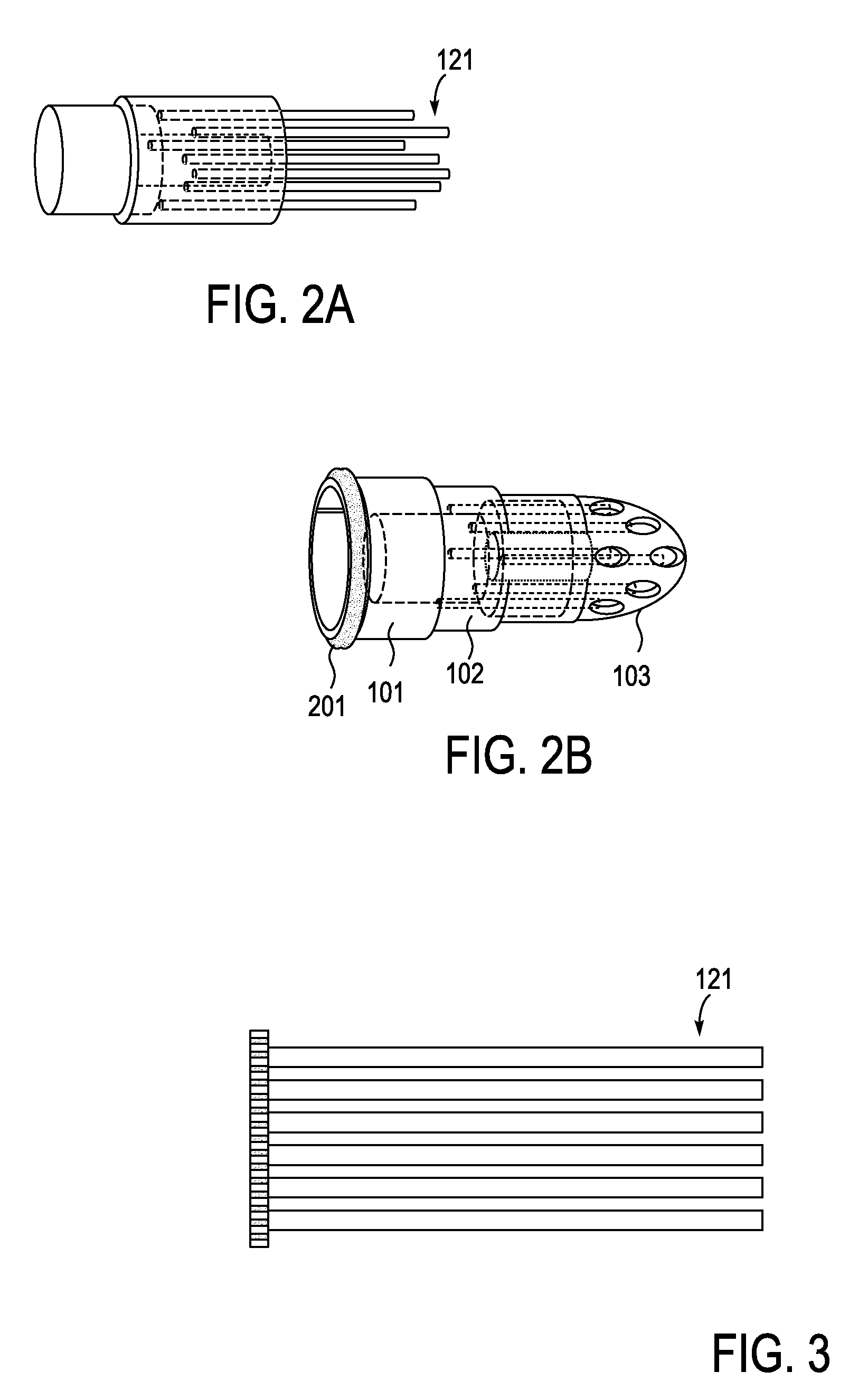
Described herein are devices, systems and methods for treating disease and / or infection by the release of silver from an implant over an extended period of time. In particular, the devices described herein may be used to treat infections such as osteomyelitis by the controlled release of silver ions from multiple sites of an extended-use implant. This implant typically includes a plurality of arms that both anchor and help distribute the released ions within the tissue. Power may be applied to release the silver ions into the tissue.
Owner:SILVER BULLET THERAPEUTICS
Bone implants for the treatment of infection
ActiveUS20110054612A1Improve adhesionPrevent leechingBone implantPharmaceutical delivery mechanismDiseaseControl release
Described herein are devices, systems and methods for treating disease and / or infection by the release of silver from an implant over an extended period of time. In particular, the devices described herein may be used to treat infections such as osteomyelitis by the controlled release of silver ions from multiple sites of an extended-use implant. This implant typically includes a plurality of arms that both anchor and help distribute the released ions within the tissue. Power may be applied to release the silver ions into the tissue.
Owner:SILVER BULLET THERAPEUTICS
Medicine controlled functional cement with calcium phosphate being as framework and its preparation method
A calcium phosphate cement with the function of controlling medicine release for repairing dysostosis, treating osteomyelitis and preventing infection and recurrence of osteoma is prepared from porous calcium phosphate cement and medicine capsules containing antibacterial medicine, antineoplastic medicine, anti-flammatory antalgic medicine, antituberculosis, etc. Its advantages are slow medicinesreleasing, and high curative effect.
Owner:EAST CHINA UNIV OF SCI & TECH
Chinese traditional medicine for treating mammary glands hyperplasia
InactiveCN101229357ARapid flow of bloodAmphibian material medical ingredientsHeavy metal active ingredientsAdditive ingredientPoultice
The invention relates to a traditional Chinese medicine for curing mammary hyperplasia, which comprises oral administration Chinese traditional pills and external application plaster. The oral administration Chinese traditional pills comprise 23 Chinese herb medicines, while the external application plaster comprises 32 Chinese herb medicines. The ingredients of the traditional Chinese medicine formula is mixed compatibly and properly; the plaster and the oral administration pills of the invention can effectively cure the mammary hyperplasia caused by various reasons and has fast efficacy, short course of treatment, high cure rate and low cost. The traditional Chinese medicine of the invention has unique healing efficacy to breast adenofibroma, breast cancer, nipple discharge, mastalgia, mastitis, breast deformity, micromazia, osteomyelitis, femoral head necrosis, various sores, lymphoid tuberculosis and eye-mouth deviation.
Owner:胡永旺
Phosphonated rifamycins and uses thereof for the prevention and treatment of bone and joint infections
InactiveUS20110178001A1High binding affinityHigh affinityAntibacterial agentsBiocideAntibiotic YJoint infections
The present invention relates to phosphonated Rifamycins, and methods of making and using such compounds. These compounds are useful as antibiotics for prophylaxis and / or the treatment of bone and joint infections, especially for the prophylaxis and / or treatment of osteomyelitis.
Owner:THE MEDICINES
Long-acting sustained-release medicaments for treating and renovating bone disease and preparation thereof
InactiveCN101244275AExtended release timeImprove adhesionInorganic non-active ingredientsSkeletal disorderMicrosphereBiocompatibility Testing
The invention discloses a long acting slow-release drug carrier material for therapy and repair of bone disease and preparation method; wherein, the components of the drug carrier material are 4-8 weight account of strontium-doped calcium polyphosphate, 1-3 weight account of chitosan drug-loaded microspheres and 1-3 weight account of chitosan; the preparation method of the compound drug carrier material for therapy of osteomyelitis and other bone diseases is that (1) the chitosan-acetic acid solution is prepared, (2) the strontium-doped calcium polyphosphate powder is put into the chitosan-acetic acid solution and dispersed evenly, (3) the prepared chitosan drug-loaded microspheres is added, (4) the cross-linking agent is added for cross-linking, (5) freezing at temperature of 2-6 DEG C is processed for 12-48 hours, and (6) heating and drying are processed at temperature of 40-100 DEG C. The drug carrier material has the advantages of being applicable to therapy and repair of osteomyelitis and other bone diseases, releasing antibiotic for a long period, bone repair function, good biocompatibility and degradability, and ideal double function of therapy and repair of osteomyelitis and other bone diseases.
Owner:SICHUAN UNIV
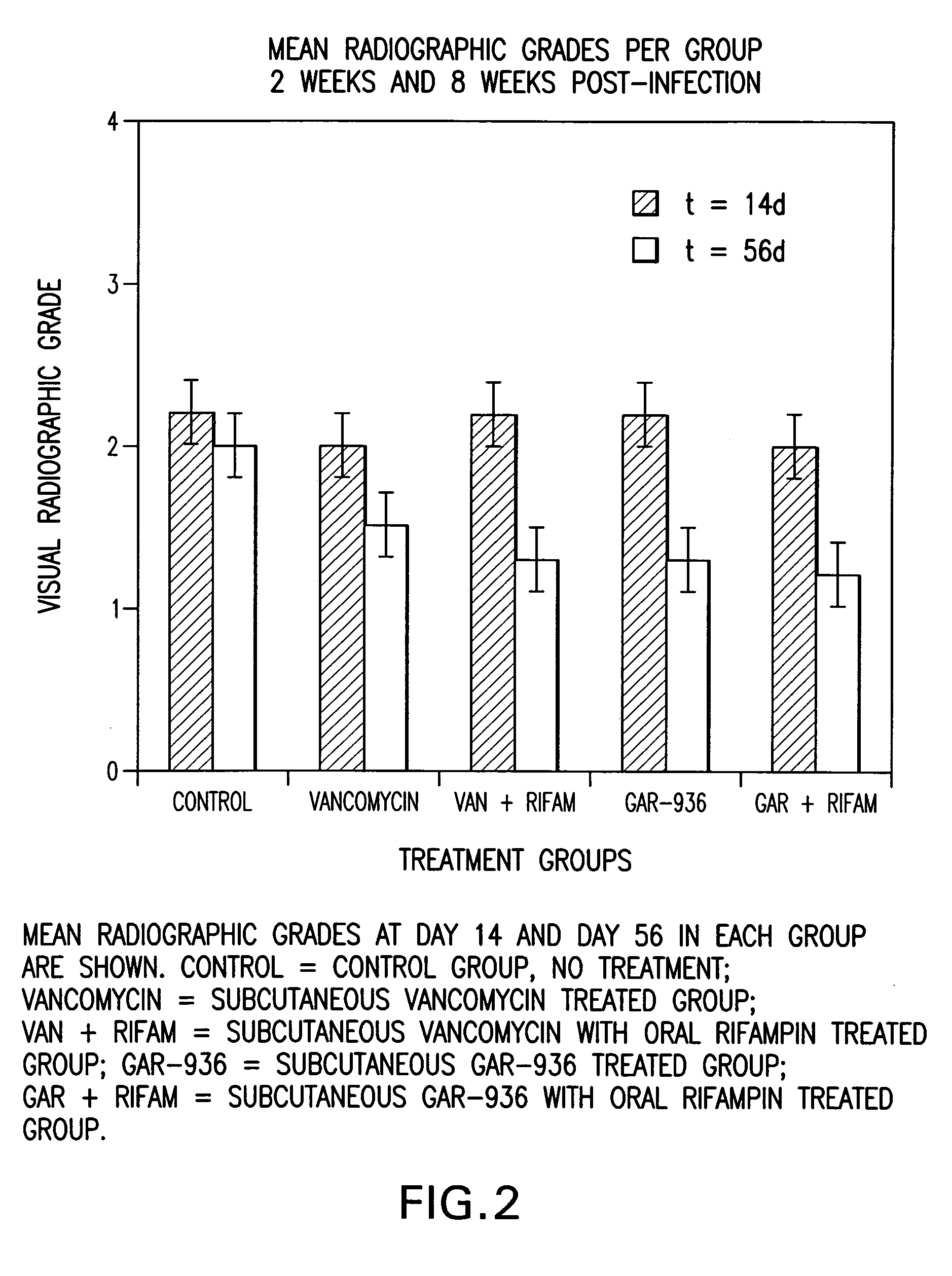
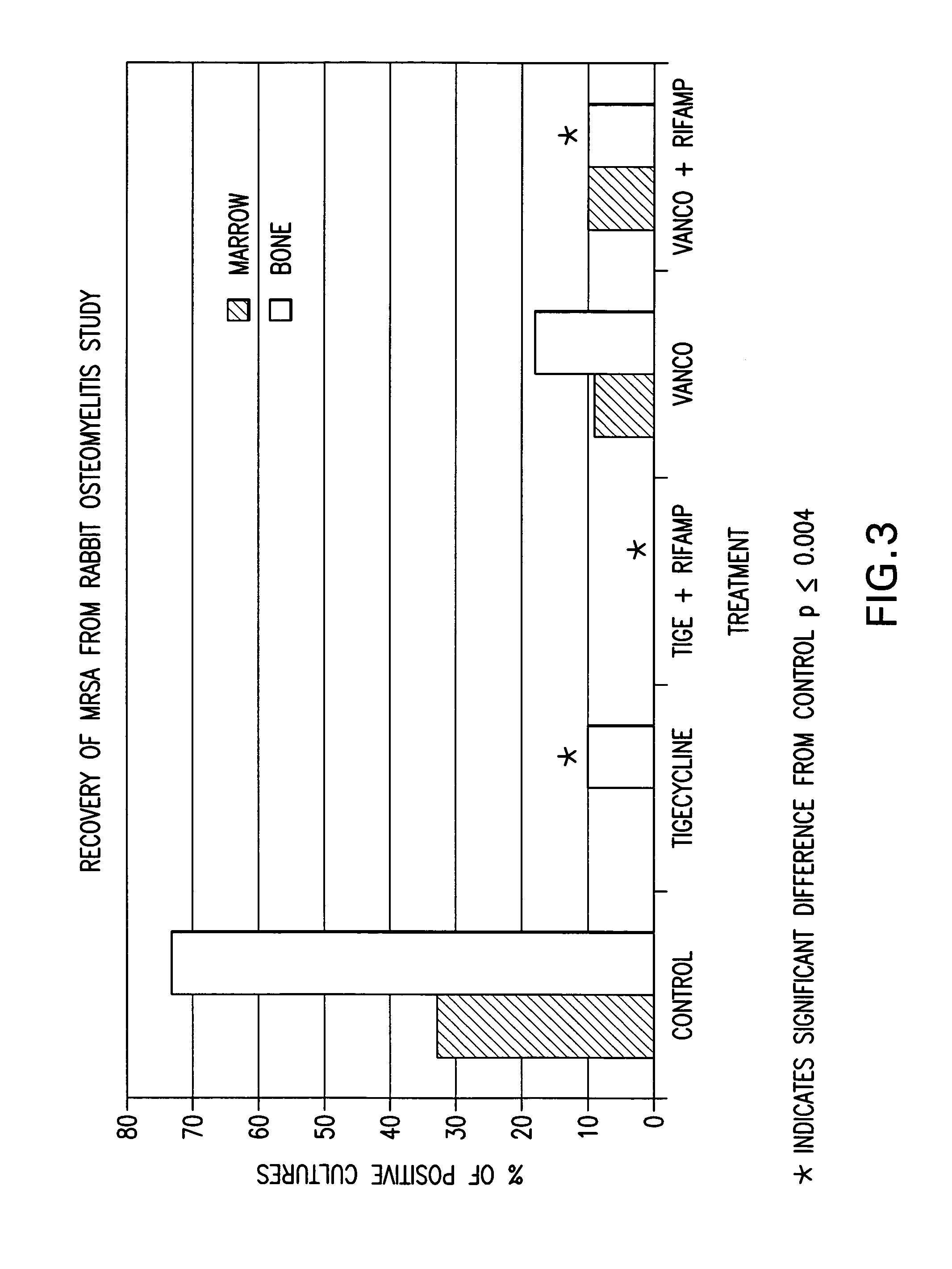
Use of tigecycline, alone, or in combination with rifampin to treat osteomyelitis and/or septic arthritis
The present invention is directed to a method for treating bone or bone marrow infections, joint infection or infection of the tissues surrounding the joint by administration of the antibiotic tigecycline alone or in combination with a rifamycin antibiotic. In a preferred embodiment the bone or bone marrow infection causes osteomyelitis. In another embodiment the joint infection or infection of the tissues surrounding the joint causes septic arthritis. The invention is also directed to manufacture of a medicament for treatment of bone and / or bone marrow infections, or joint infections and / or infections in tissues surrounding the joint with tigecycline alone or in combination with rifampin.
Owner:WYETH +1
Flooring challenge systems for culling poultry
Flooring challenge systems for culling poultry that induce lameness attributable to osteochondrosis and osteomyelitis of the proximal femur and tibia in poultry. The flooring challenge systems induce unstable or insecure footing for poultry reared in pens by adding torque, stress and strain on key leg joints in order to exacerbate and accelerate the development of bacterial chondronecrosis with osteomyelitis lesions and lameness. The flooring challenge systems utilize one or more portable panels or sections that can be constructed in a wide range of sizes and configurations for installation in commercial poultry pens. In addition, the flooring challenge systems may utilize a device suspended a predetermined distance above the apex of the flooring challenge panel to subject the poultry's legs to asymmetric twisting and enhanced instability by forcing the birds to straddle opposing slopes near the apex of the flooring challenge panel.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
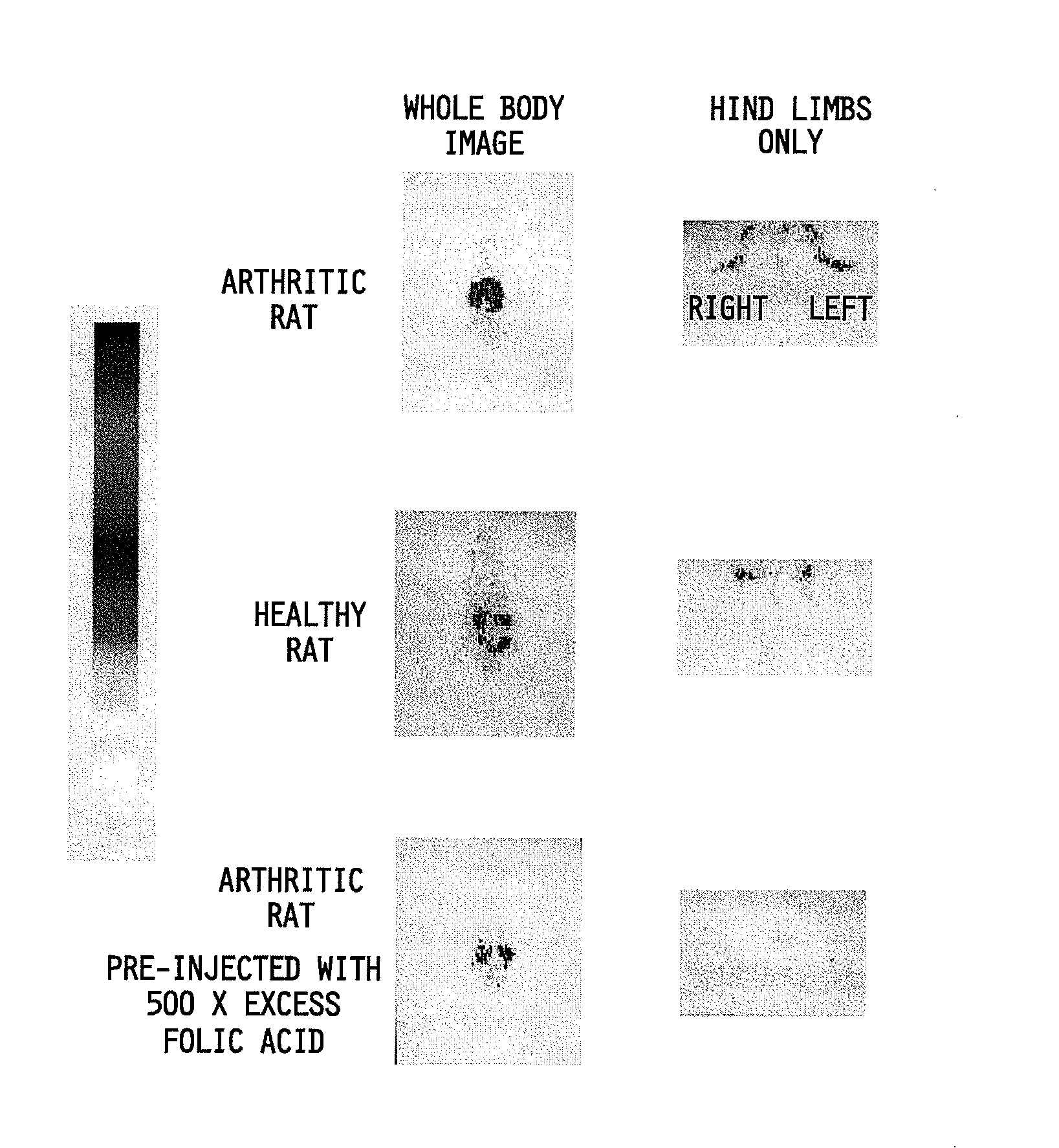
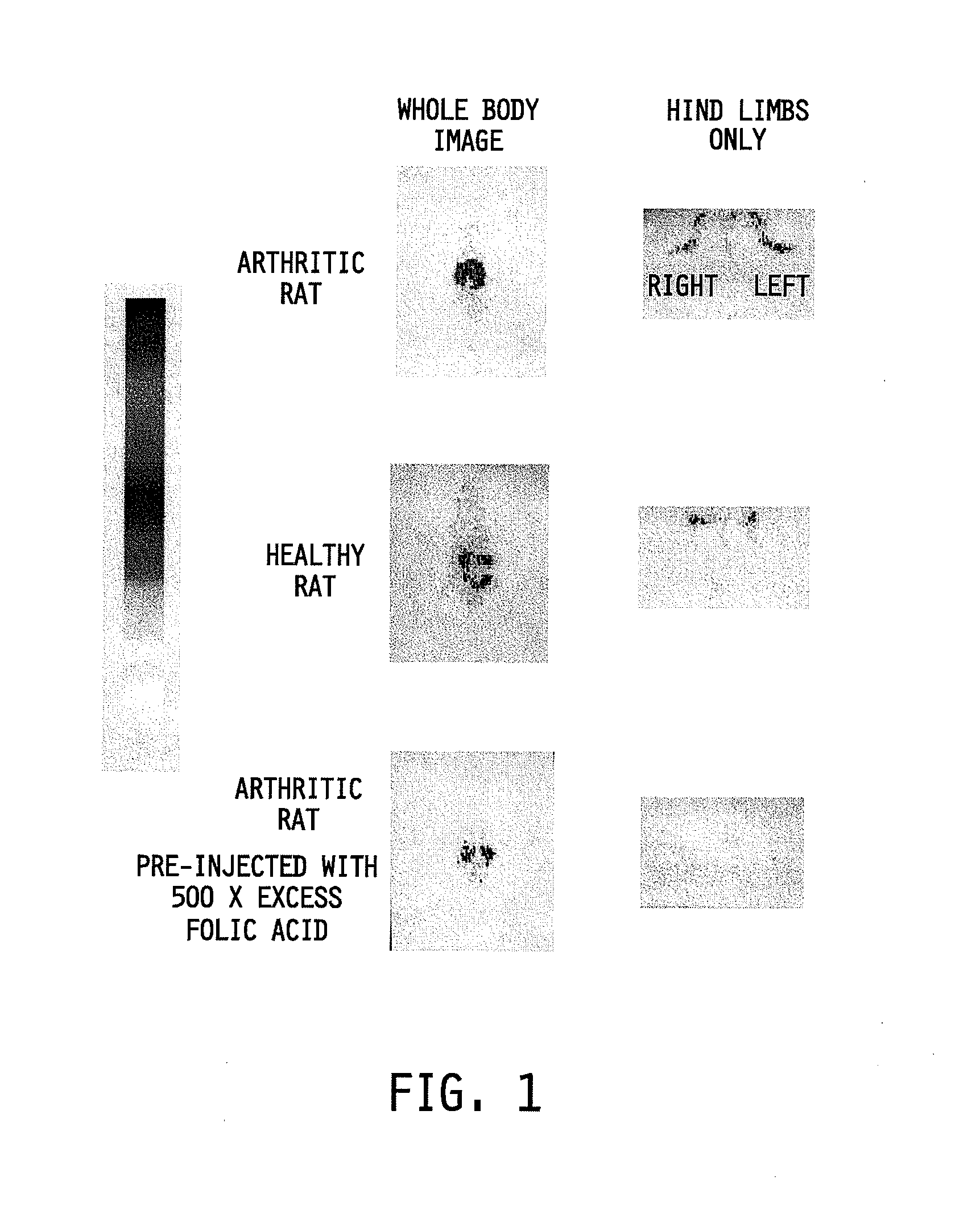
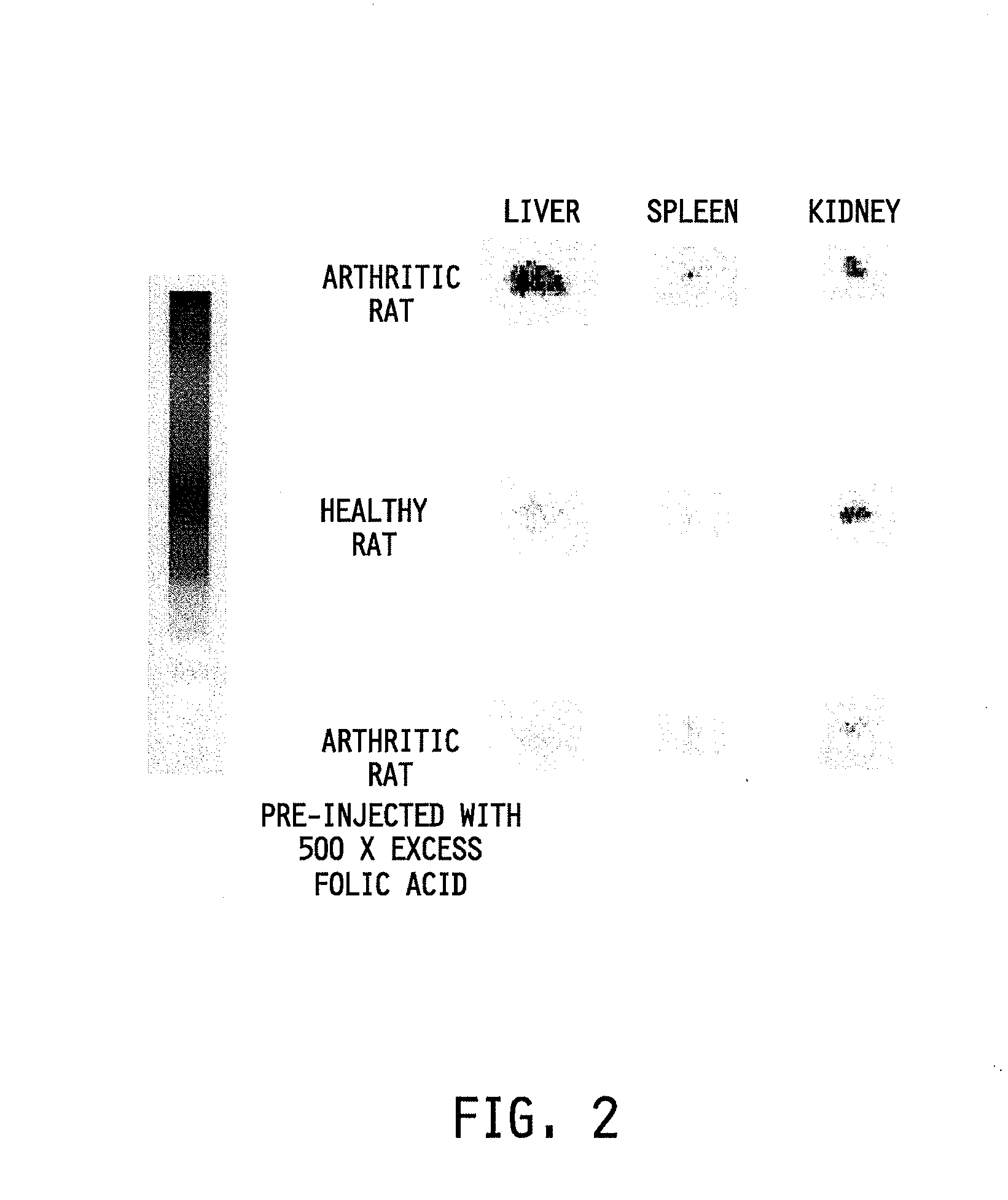
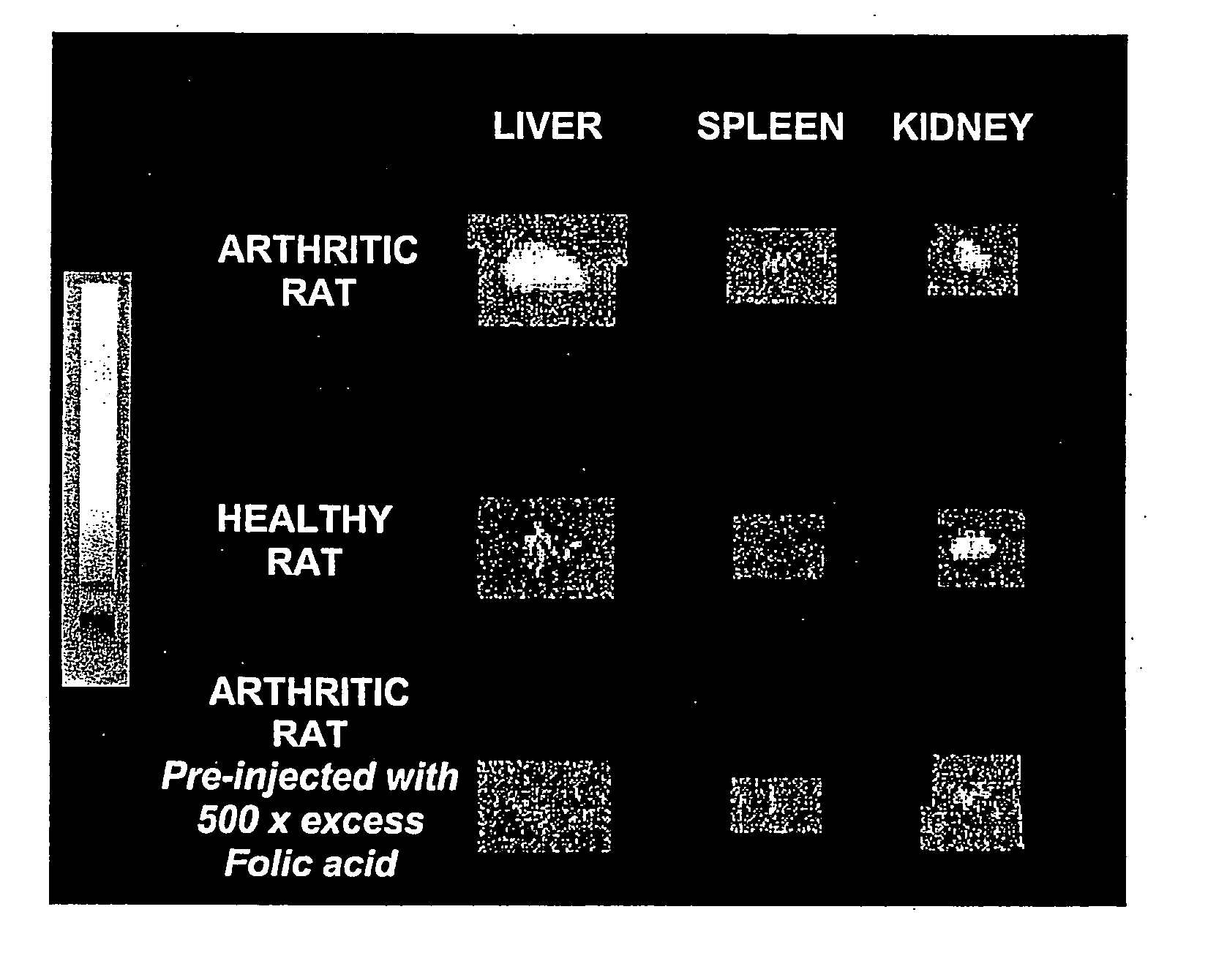
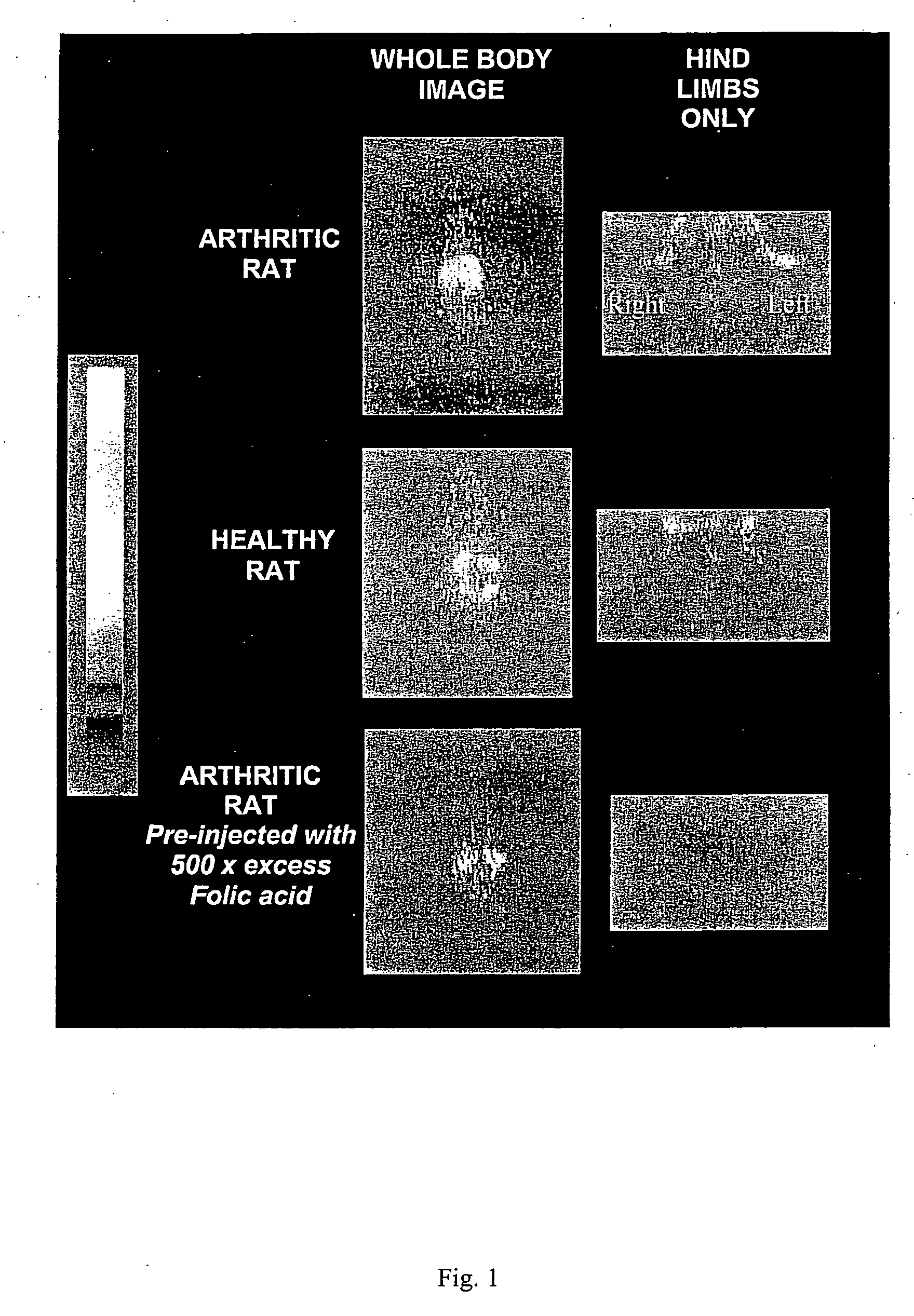
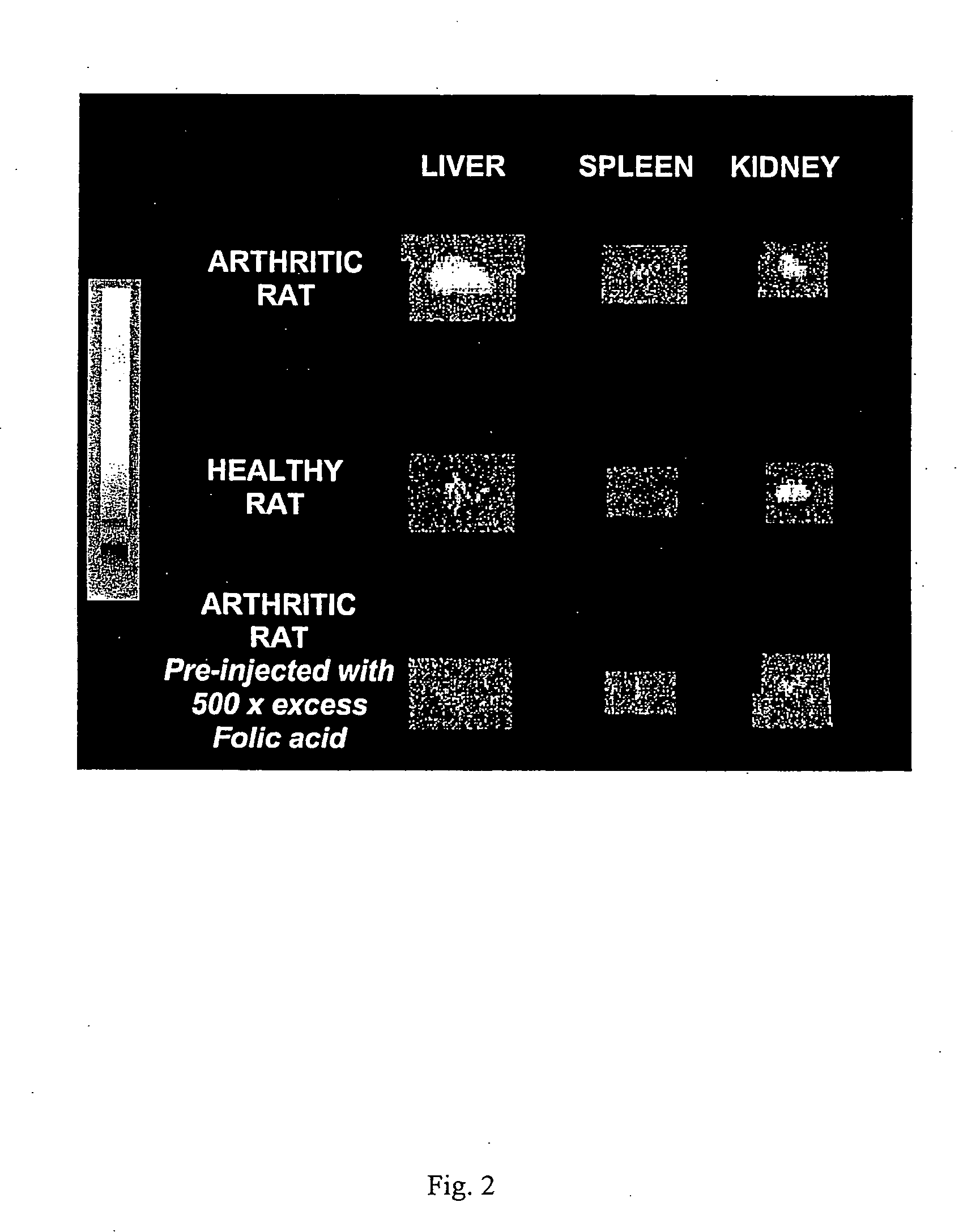
Treatment of osteomyelitis with radiopharmaceuticals
InactiveUS7045116B2Osteomyelitis is reducedAntipyreticAnalgesicsNuclear medicineTuberculous osteomyelitis
This invention relates to medical uses of radiopharmaceuticals. Specifically, the present invention relates to the use of radiopharmaceuticals to treat osteomyelitis. The present invention provides improved system and methods of for the direct delivery of radiopharmaceuticals to the site of osteomyelitis.
Owner:DOW GLOBAL TECH LLC
Broad-spectrum epidemic preventing antiviral traditional Chinese medicine composition
ActiveCN103536884AImprove immunityDrug resistanceAntibacterial agentsAntiviralsTreatment effectLicorice roots
The invention discloses a broad-spectrum epidemic preventing antiviral traditional Chinese medicine composition which comprises the following traditional Chinese medicine components: honey-fried licorice roots, the fruits of Chinese wolfberry, lucid ganoderma, ginseng, rheum officinale, isatis roots, Astragalus membranaceus, honeysuckle and the like. The water decoction of the medicine disclosed by the invention is remarkable in effect for preventing and treating viral diseases. When viral diseases break out on a large scale, the water decoction disclosed by the invention can be taken in advance to have the effects of building the body and enhancing the immunity as well as resisting invasion of viruses. Once the patient suffers from viral diseases, particularly flu, mumps, cytomegalovirus retinitis and osteomyelitis, the water decoction disclosed by the invention is taken and further has remarkable treatment effect. Furthermore, the viruses do not easily generate drug resistance and tolerance, therefore, the traditional Chinese medicine composition is the first choice for broad spectrum, epidemic prevention and virus prevention.
Owner:袁展
Chinese medicine ointment for treating arthrosis
InactiveCN1401360AReduce the burden of medical expensesNo painAnthropod material medical ingredientsAerosol deliveryDiseaseCure rate
A Chinese medicine in the form of ointment for treating arthropathy, such as cervical spondylopathy, lumbar disease, osteomyelitis, etc. is prepared from more than 40 Chinese-medicinal materials including trogopterus dung, astragalus root, leech, notoginseng, etc. Its advantages are high cure rate (more than 90%) and low recurrence rate.
Owner:任景兰 +1
Osteoprotegerin variant proteins
InactiveUS20060189528A1Reduced binding affinityReduce capacityNervous disorderPeptide/protein ingredientsPAGET'S BONE DISEASEWild type
The present invention relates to novel osteoprotegerin variant proteins (OVPs) that demonstrate reduce binding affinity for their ligand TRAIL when compared to wild-type osteoprotegerin. Nucleic acids which encode these OVPs are also provided. Recombinant vectors and host cells expressing these OVPs are also encompassed as are methods of producing recombinant OVPs. The present invention also relates to compositions comprising these OVPs, and to methods of treating bone diseases characterised by increased bone turnover and / or loss. The OVPs of the invention are useful for preventing bone resorption and may be used to treat any condition resulting in abnormal bone turnover or bone loss such as osteoporosis, hypercalcemia, Paget's disease of bone, multiple myeloma, bone cancer and bone loss due to rheumatoid arthritis or osteomyelitis, and the like.
Owner:CEPHALON AUSTRALIA
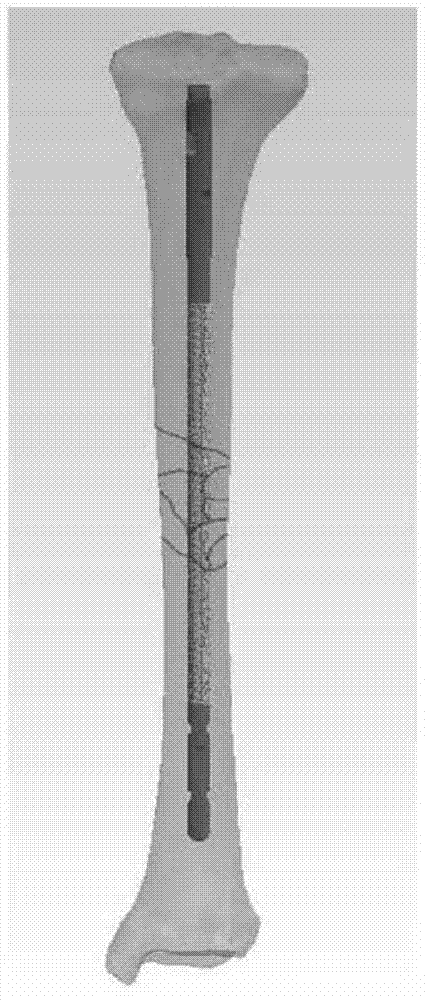
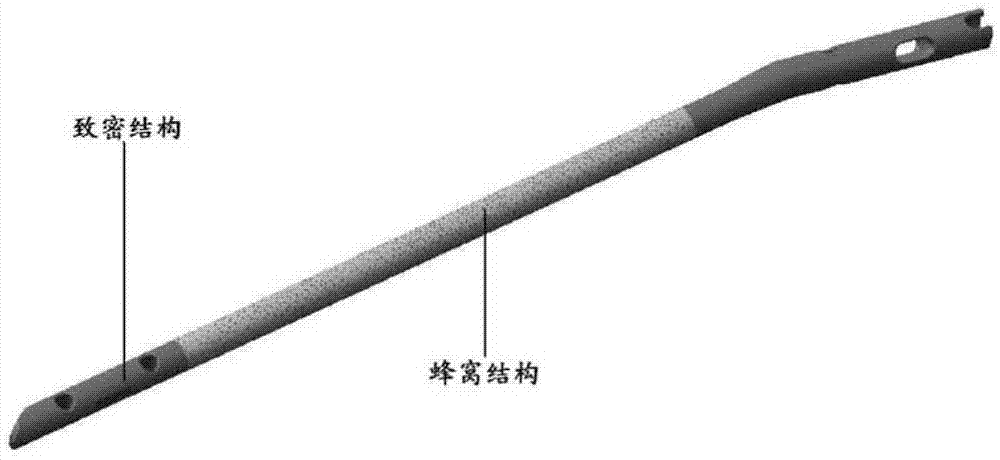
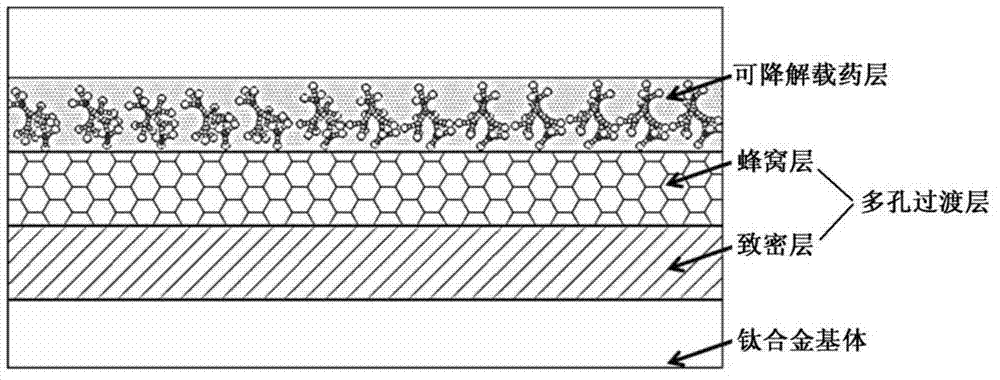
Intramedullary nail having multifunctional coating and preparation method
The invention relates to the medical instrument field, especially relates to the orthopaedics titanium alloy internal fixation field. The invention provides an intramedullary nail having a multifunctional coating and a preparation method. The intramedullary nail comprises a multifunctional composite medicament coating slow release system, and the slow release system comprises a porous transition layer and a degradable coating containing the medicament. The intramedullary nail integrates the functions of preventing, treating infection and internal fixation as one, and is mainly used for treating adaptation diseases such as open fracture and osteomyelitis, and has the effect for degrading and slowly releasing the medicines.
Owner:SUZHOU MINIMALLY INVASIVE SPINAL TRAUMA MEDICAL TECH CO LTD
Pain relieving fitness paste and production method thereof
InactiveCN102824452AThe formula is scientific and reasonableKeep active ingredientsHydroxy compound active ingredientsAntipyreticSalvia miltiorrhizaMENTHOL CRYSTALS
The invention relates to a pain relieving fitness paste and a production method thereof which are mainly used for providing medication for treating the pain caused by osteomyelitis, arthritis and hyperosteogeny. The pain relieving fitness paste is prepared from 90-120g of radix salviae miltiorrhizae, 90-120g of safflower, 90-120g of caulis spatholobi, 90-120g of fructus gardeniae, 45-65g of radix clematidis, 45-65g of radix angelicae pubescentis, 45-65g of pricklyash peel, 130-165g of borneol, 90-120g of camphora and 90-120g of menthol crystal. The production method comprises the following steps of: grinding the radix salviae miltiorrhizae, safflower, caulis spatholobi, fructus gardeniae, radix clematidis, radix angelicae pubescentis and pricklyash peel into coarse powder; percolating the coarse powder with ethanol; concentrating the percolate into extract; extracting the dregs with gasoline to obtain extraction oil; preparing rubber paste, rosin, zinc oxide, wool fat, yellow vaseline, liquid paraffin, gasoline or SIS, liquid paraffin, glycerol ester of rosin and 2,6-butylated hydroxytoluene into a matrix; adding the extract, extraction oil, borneol, camphora and menthol crystal; and smearing the obtained product on the base cloth, covering with an anti-sticking layer, and slicing. Through the invention, the formula is scientific and reasonable, the active ingredients are maintained to the greatest degree, and the curative effect is improved.
Owner:吕秀兰 +1
Application of tanshinone óÄA in pharmacy
InactiveCN1631364AEnhance pharmacological effectsSufficient extractionOrganic active ingredientsSkeletal disorderPharmacyMedicine
The invention relates to the use of tanshinone IIA in the preparation of medicaments for treating atherosclerosis, myocardial infarction, coronary heart disease, tumor, purulency osteomyelitis and tonsillitis. The tanshinone IIA of the invention can be prepared into various dosage types.
Owner:昆明希捷医药研发有限公司
Medicine for treating bone tuberculosis, osteomyelitis, synovitis and angeitis
InactiveCN1634312AHigh cure rateShort treatment periodAntibacterial agentsAntipyreticBletilla striataSYNOVIAL SWELLING
The invention discloses a medicine for treating bone tuberculosis, osteomyelitis, synovitis and angeitis which comprises the raw material of Chinese medicinal herbs including radix adenophorae, Chinese angelica root, Ligusticum wallichii, Poria cocos, bitter orange, white atractylodes rhizome, atractylodes rhizome, root of herbaceous peony, rhizoma dioscoreae, astragalus root, drynaria, psoralea fruit, dipsacus root, rhizoma corydalis, cyperus tuber, tortoiseshell, bletilla striata, Poria cocos, silver flower, chicken's gizzard-skin, malt, magnolia obavata, amomum fruit, dried orange peel, job's tears, and Chinese honeylocust spine.
Owner:杨琰
Phosphonated Fluoroquinolones, Antibacterial Analogs Thereof, and Methods for the Prevention and Treatment of Bone and Joint Infections
InactiveUS20080287396A1High binding affinityHigh affinityAntibacterial agentsBiocideFluoro quinolonesAntibiotic Y
The present invention relates to phosphonated fluoroquinolones, antibacterial analogs thereof, and methods of using such compounds. These compounds are useful as antibiotics for prevention and / or the treatment of bone and joint infections, especially for the prevention and / or treatment of osteomyelitis.
Owner:TARGANTA THERAPEUTICS INC
Cefpodoxime proxetil submicron emulsion solid preparation and novel application thereof
InactiveCN101708166AImprove stabilityImprove solubilityAntibacterial agentsOrganic active ingredientsEmulsionBioavailability
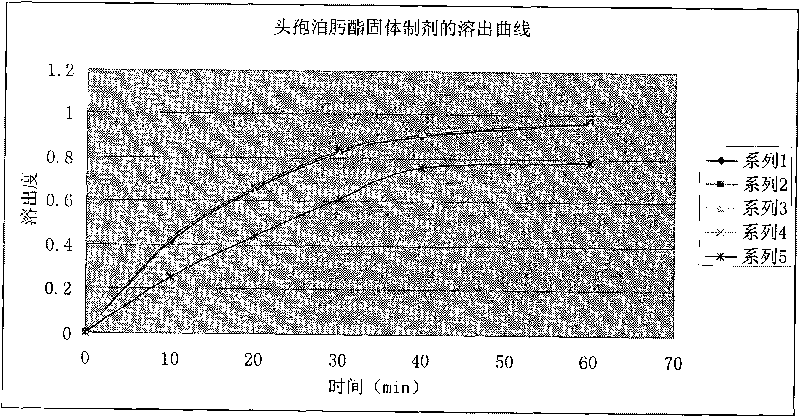
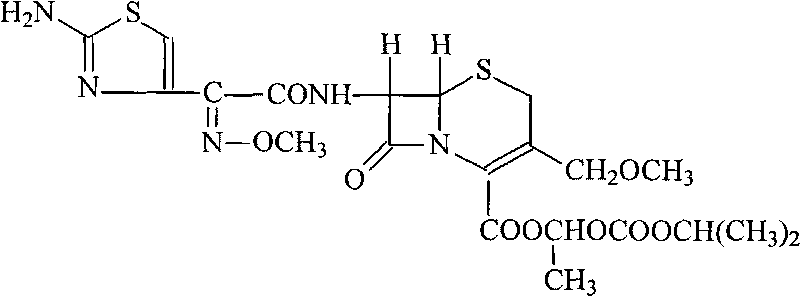
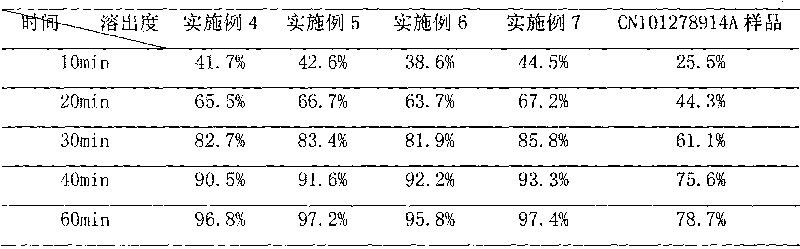
The invention discloses a cefpodoxime proxetil submicron emulsion solid preparation and a novel application thereof, particularly a cefpodoxime proxetil solid preparation which is subjected to micro-emulsification and a novel application thereof. In the invention, the micro-emulsification technology is applied to process cefpodoxime proxetil raw materials so as to obtain a cefpodoxime proxetil submicron emulsion with excellent performance, the stability of the cefpodoxime proxetil is improved and the dissolution rate of the cefpodoxime proxetil preparation is obviously improved, so that the cefpodoxime proxetil submicron emulsion solid preparation has better bioavailability and can be used for preparing a medicament for treating osteomyelitis of jaws.
Owner:HAINAN MEIDA PHARMA
Local sustained release preparation for preventing and treating osteomyelitis, preparation method and application thereof
InactiveCN101618209AImprove the bactericidal effectImprove release abilityAntibacterial agentsPeptide/protein ingredientsMicrospherePhosphoric acid
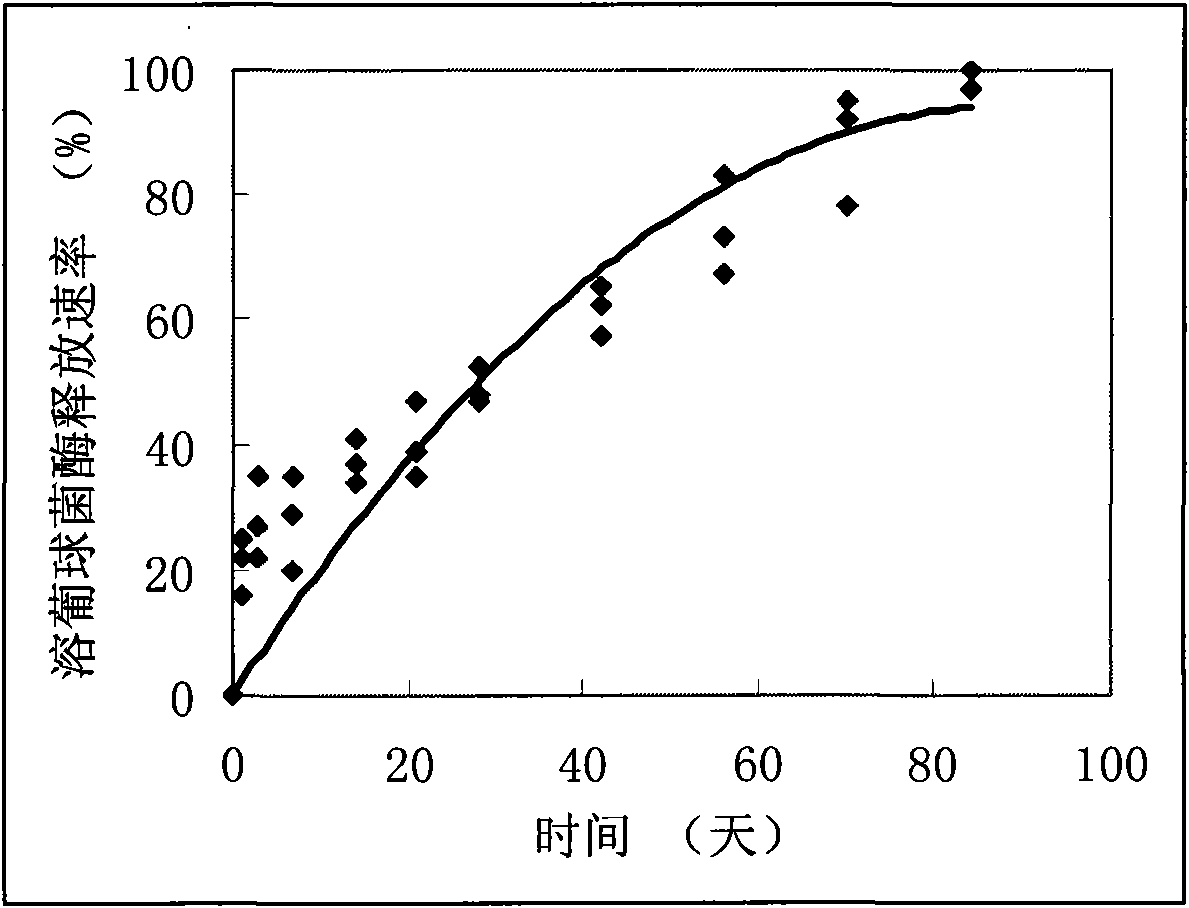
The invention discloses a local sustained release preparation for preventing and treating osteomyelitis, a preparation method and application thereof. The preparation comprises the following components in percentage by weight: 0.01 to 1 percent of staphylococcus lysozyme, 1 to 20 percent of polylactic acid (PLA), 0.5 to 10 percent of polylactide-co-glycolide (PLGA), and 70 to 90 percent of calcium phosphate cement (CPC). The preparation takes the staphylococcus lysozyme as a bactericidal active component to obtain PLA-PLGA polymer microspheres containing the staphylococcus lysozyme through supercritical fluid microparticle preparation technology, and the PLA-PLGA polymer microspheres are compounded with the calcium phosphate cement (CPC) to obtain a novel sustained release degradable antibacterial composite artificial bone carrying staphylococcus lysozyme microspheres. The preparation has good physical and chemical properties and strong sterilization effect, ensures that the in vitro medicament release process is long up to three months, has steady release rate, and can maintain steady and long-acting release at administration positions.
Owner:SHANGHAI HI TECH UNITED BIO TECHCAL RES
Injectable calcium sulphate-based sustained-release implantation component containing medicine-carrying particle and application
InactiveCN101485904AOvercome the defect of loose contactSuitable for growthSkeletal disorderPharmaceutical non-active ingredientsFiberMicroparticle
The invention relates to a medicine-carried injectable calcium sulfate based slow-release implant composition and application thereof. The slow-release implant composition adopts a component A and a component B, wherein the component A comprises 100 portions of semi-hydrated sulfuric acid, 0.1 to 10 portions of nucleater, 0.1 to 5 portions of plasticizer, 0 to 5 portions of surfactant and 2 to 5 portions of medicine-carrying particles, and the medicine-containing mass concentration is between 4 and 20 percent; and the component B is a diluent, and the weight of the diluent is 30 to 60 portions as calculated by 100 portions of the semi-hydrated sulfuric acid. The medicine-carried particle injectable calcium sulfate based slow-release implant system has the advantages that: the slow-release implant system can inject local medicine-release implant into focuses and make the implant formed without operation, overcome the defect that general preformed implant materials do not closely contact circumferential bone tissues, and prevent encapsulation of fiber tissues; due to the osteoacusis function of calcium sulfate, the slow-release implant system is more suitable for growth and repair of the bone tissues; and due to unique medicine-carried particles, the slow-release implant system can realize local medicine release, is stable to release medicines, has long medicine effect time and high medicine utilization rate, and is suitable for treating bone defect and the like caused by osteomyelitis, osteocarcinoma and the like.
Owner:TIANJIN UNIV
Chinese traditional medicine agent for treating osteomyelitis
InactiveCN101062235AScientific and reasonable configurationGood treatment effectAntipyreticAnalgesicsTherapeutic effectDrynaria
The invention relates to a traditional Chinese medicinal composition for treating medullitis which comprises the following raw materials (by weight portion): astragalus root, poria cocos, root of red rooted saliva, dried orange peel, honeysuckle flower, Chinese yam each 20-30g, dandehon herb 40-50g, achyranthes and cyathula root, corktree bark, Chinese mugwort leafmulberry leaf, Philippine violet herb each 15-20g, Chinese angelica root, gynostemma pentaphylla and drynaria each 10-15g.
Owner:隋朝霞
External ointment for treating various inflammations and scabies and malignant sores and preparation method thereof
The invention provides external ointment for treating various inflammations and scabies and malignant sores, which is characterized by comprising the following components in percentage by weight: 5 to 25 percent of mongolian dandelion herb, 1 to 25 percent of lightyellow sophora root, 1 to 10 percent of borneol, 5 to 25 percent of amur corktree bark and 5 to 40 percent of honeysuckle flower. A preparation method comprises the following steps of: removing impurities, cleaning, drying, grinding, sieving and decocting into the ointment. The ointment has the effects of clearing pyretic toxicity, subduing swelling and stopping pain, eliminating malignancy and tuberculosis, treating acute mammitis, mumps, acute conjunctivitis, acute tonsillitis, adenolymphitis, ulcer and furunculosis, haemorrhoids, ulcers and pyogenic infections, male phallical abscess, herpes simplex and blistering, eczema, scabies, furunculosis and sores, acute mastitis swelling, sores, osteomyelitis, scabies and malignant sores, pruritus in erosion of vulva, scrofula, accumulation in abdominal mass and pustule sores, treating patients who cannot suffer from sore pain, eliminating skin blood-heat and the like.
Owner:TIANJIN DEV ZONE TAIREN BIOTECH
Poly acid anhydride for release-controlled medicine carrier and method for producing the same
InactiveCN1544508AReduce stimulationLong release periodPharmaceutical non-active ingredientsImmunological disordersDiseaseSynthesis methods
The invention discloses a process for preparing polyanlydrides, carriers for hydrophilous antiphlogistic drug and antitumor agent by using binary aliphatic acid and dimer acid copolymerization, because the use of diacid with longer carbon chain as monomer, the degradation speed of the polyanlydride material is slow, and the medicinal release cycle is longer, thus is suitable for the local slow release administration treatment for osteomyelitis and solid tumor diseases.
Owner:HUAZHONG UNIV OF SCI & TECH
Black purple Tuowusu and medical application in restraining grampostive bacteria
This invention relates to Ligulatrovine A separated from Ligularia actroviolacea that can prevent and treat diseases related to Staphylococcus aureus and beta hemolytic streptococcus, its pharmaceutical salts and drug composition. Ligulatrovine A has significant inhibitive effect on Gram-positive bacteria, and can prevent and treat diseases such as boil, pustule, pneumonia, osteomyelitis, acute myocarditis, endocarditis, meningitis, mastitis, cystitis, pelvic inflammation, urinary inflammation, prostatitis, bacteremia, or abscess in muscle, skin, urogential region or central nervous system caused by Gram-positive bacteria infection.
Owner:WENZHOU MEDICAL UNIV
Methods and Pharmaceutical Compositions for the Treatment of Bone Density Related Diseases
InactiveUS20130195863A1Decreased bone mineral densityIncreasing bone mineral densityOrganic active ingredientsPeptide/protein ingredientsGhosal hematodiaphyseal dysplasiaBone density
The invention relates to methods and pharmaceutical compositions for the treatment of bone density related diseases. More particularly, the present invention relates to a ROBO1 modulator for use in a method for the treatment of a bone mineral density related disease in a subject. In a particular embodiment the ROBO1 modulator is selected from the group consisting of small organic molecules, antibodies, aptamers or polypeptides. In another particular embodiment said bone mineral density related disease is selected from the group consisting of ghosal hematodiaphyseal dysplasia syndrome (GHDD), osteoporosis, osteoporosis associated to pseudoglioma, osteoporosis and oculocutaneous hypopigmentation syndrome, osteoporosis due to endocrinological dysfunction, osteogenesis imperfecta osteopenia, Paget's disease, osteomyelitis, hypercalcemia, osteonecrosis, hyperparathyroidism, lytic bone metastases, periodontitis, bone loss due to immobilization and osteoporosis associated with a disease selected from the group consisting of cachexia, anorexia, alopecia, rheumatoid arthritis, psoriatic arthritis, psoriasis, and inflammatory bowel disease.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
Exosome protein for osteosarcoma diagnosis and instant detecting method thereof
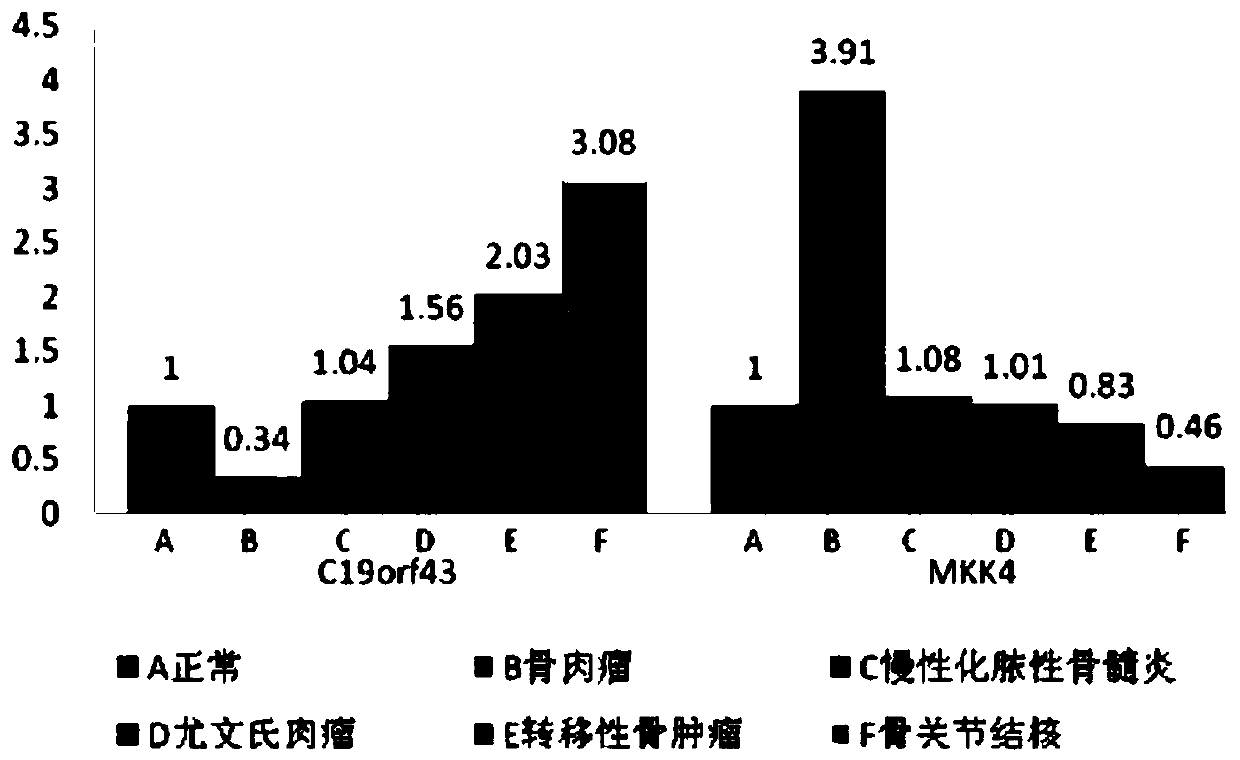
The invention discloses use of a reagent for detecting the expression level of a C19orf43 protein from a serum exosome as a marker for diagnosing osteosarcoma. The maker can realize reliable diagnosis, particularly realizes distinguishing of osteosarcoma, chronic pyogenic osteomyelitis, Ewing's sarcoma, metastatic tumor of bone and osteoarticular tuberculosis. Furthermore the invention provides aninstant detecting platform of the exosome protein for detecting the marker. The method has advantages of high efficiency, simple operation, low cost and high clinical auxiliary diagnosis value.
Owner:杭州多泰科技有限公司
Preparation method of sanguisorba antiphlogistic and analgesic dressing
InactiveCN101869720AAnti-inflammatoryHas antibacterial propertiesAbsorbent padsBandagesMedicineAppendicitis
The invention discloses a preparation method of a sanguisorba antiphlogistic and analgesic dressing, which comprises the following steps: 1. taking sanguisorba (long leaves and lanate velamen) velamen, washing and mashing; 2. taking a proper amount of tung oil, adding into the mashed sanguisorba velamen, and uniformly stirring into paste to prepare the sanguisorba antiphlogistic and analgesic dressing. The sanguisorba antiphlogistic and analgesic dressing is used for treating boils, carbuncles, furuncles, nameless sores or boils, mastitis, parotitis, boils, scrofula, lymphadenectasis, osteomyelitis, chronic appendicitis, injuries from falls and infection of various inflammations when externally applied and also can be used for burns and scalds.
Owner:施青春
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com