Diagnosis of macrophage mediated disease
a macrophage and disease technology, applied in immunology disorders, therapy, antibody medical ingredients, etc., can solve the problems of cartilage deterioration and bone erosion, degradation of pathogens, and consequent destruction of joint integrity, so as to reduce liver uptake, less inflamed, and less inflamed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials
[0047] EC20 (a folate-linked chelator 99mTc), EC28 (the same 99mTc chelate complex without folate), and folate-fluorescein isothiocyanate (folate-FITC) were gifts from Endocyte, Inc. (West Lafayette, Ind.). Heat-killed Mycoplasma butericum was purchased from BD Biosciences (Sparks, Md.). Folic acid, light mineral oil, clodronate, collagenase-A, and streptavidin-R-phycoerythrin were obtained from Sigma Chemical Co. (St. Louis, Mo.), and Dubelco's Modified Eagle Medium (DMEM) was from Gibco-BRL (Gathersberg, Md.). 3H-folic acid was obtained from American Radiolabeled Chemicals, Inc. (St. Louis, Mo.) and Microcon®-30 membranes were purchased from Millipore Corp. (Bedford, Mass.). RK-4-biotin and ED2-R-phycoerythrin antibodies were acquired from Bachem Biosciences, Inc. (Philadelphia, Pa.) and Accurate Chemical and Scientific Corp. (Westbury, N.Y.), respectively.
example 2
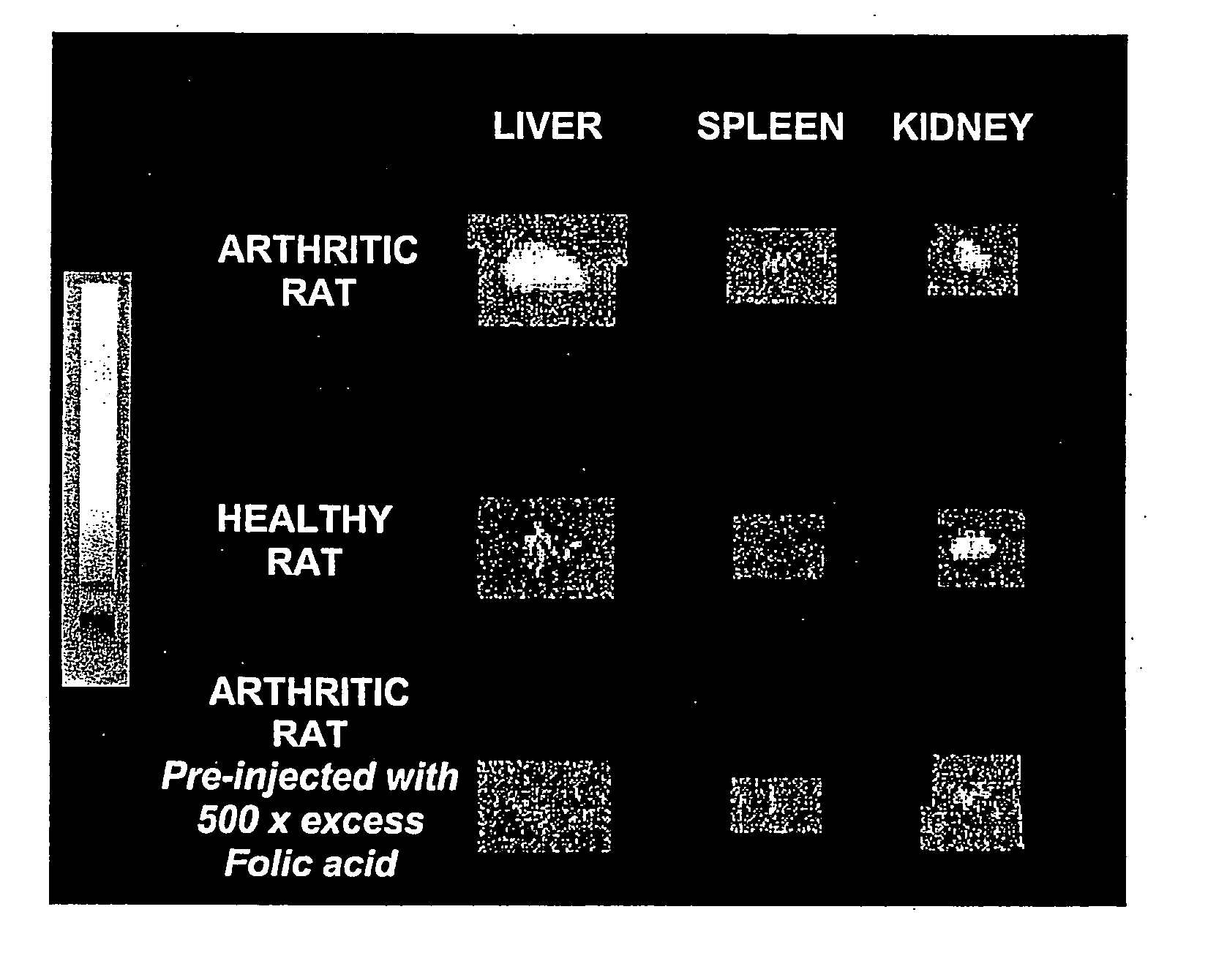
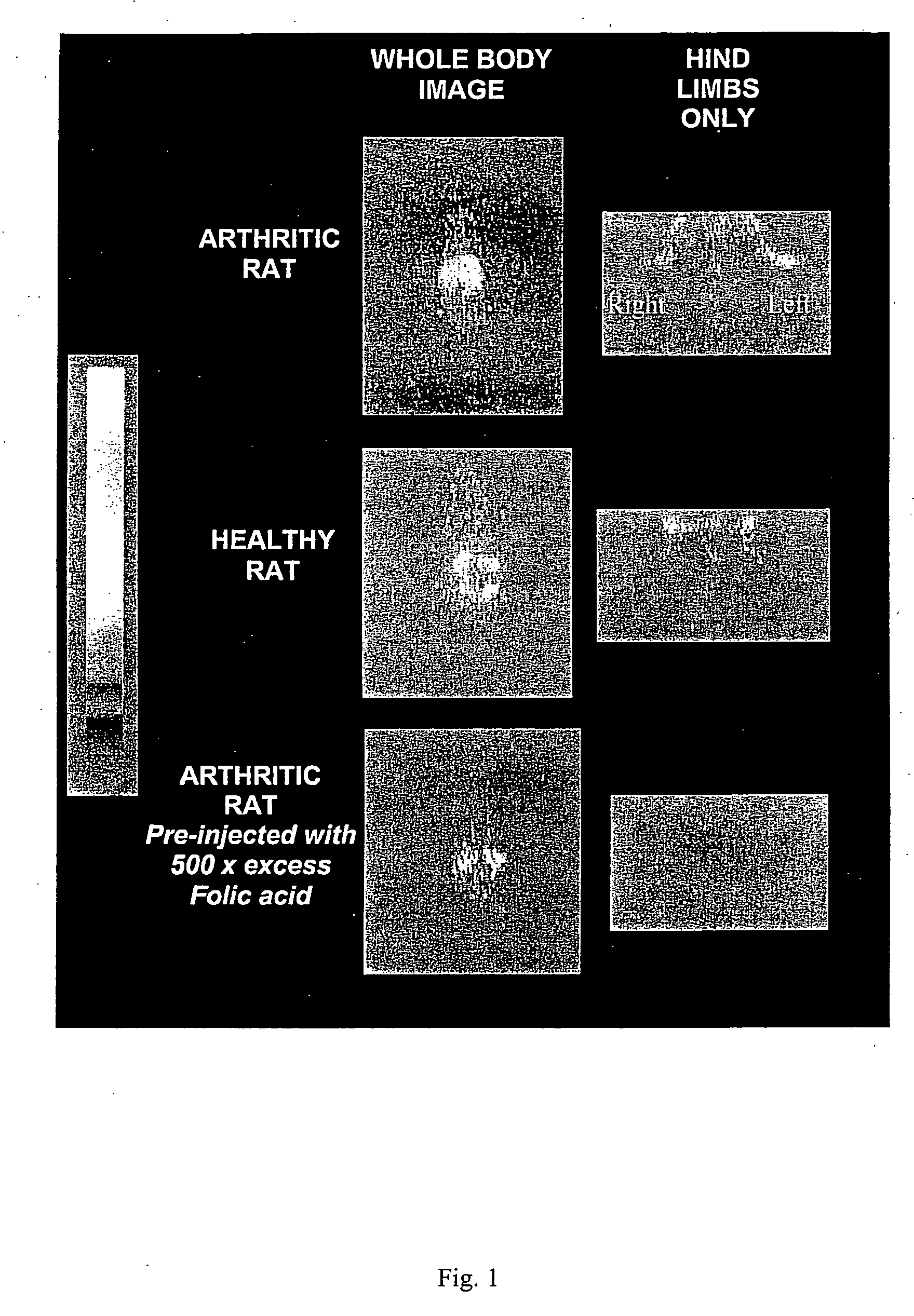
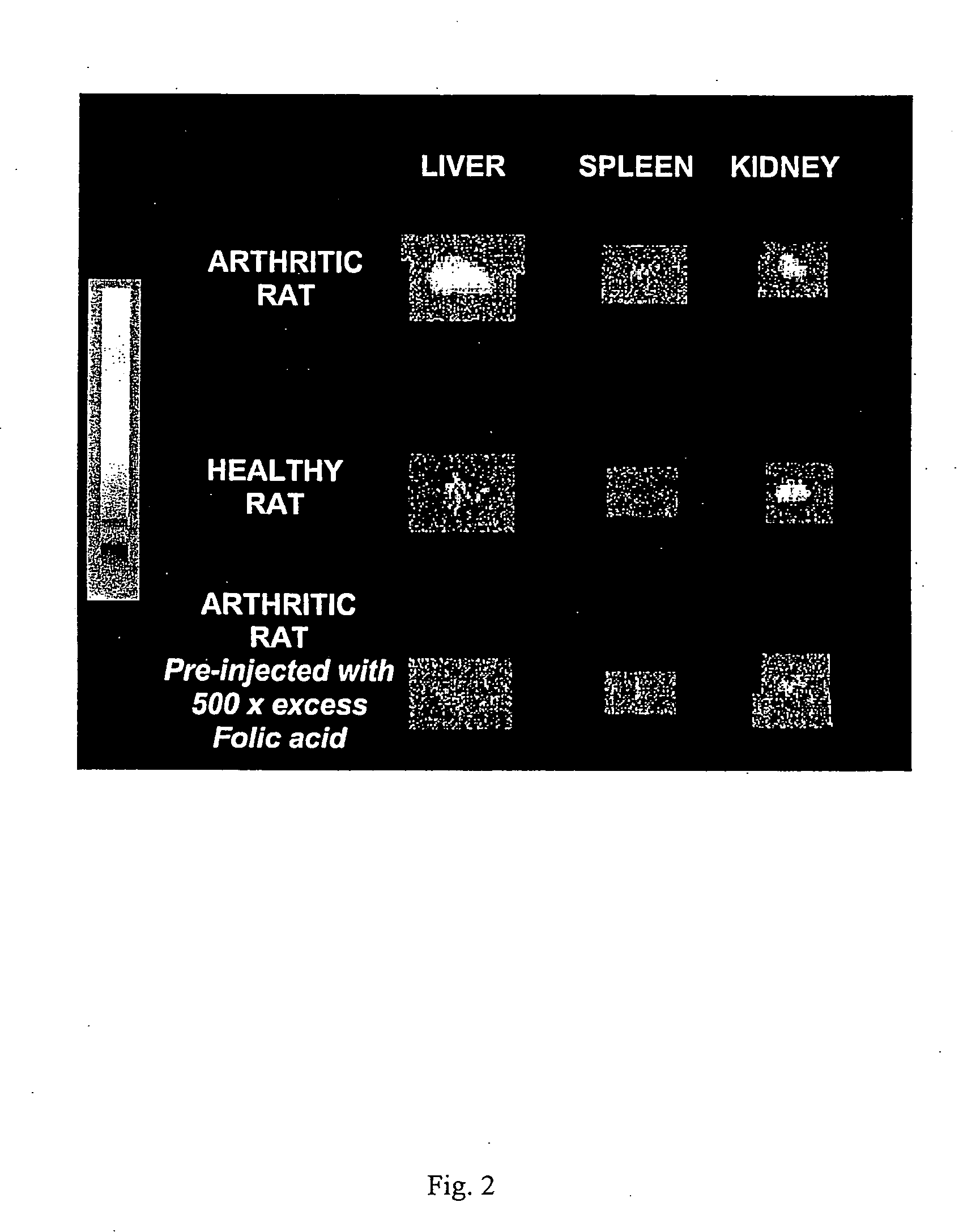
[0048] Arthritis was induced in 150-200 g female Lewis rats (Charles River Laboratories, Inc., Wilmington, Mass.), n=4 / dose group. Briefly, 0.5 mg of heat-killed Mycoplasma butericum, suspended in mineral oil (5 mg / ml), was injected on day 0 into the left hind foot of rats following anesthesia with ketamine and xylazine. Disease was allowed to progress for 21 days, and animals were weighed on a daily basis to ensure the status of their health. All treated animals developed arthritis, as evidenced by dramatic swelling in the injected paw, progressive swelling in all noninjected limbs due to the systemic progression of arthritis, and radiographic analysis of affected limbs. All rats were maintained on a folate-deficient diet (DYETS, Inc., Bethlehem, Pa.) for 3 weeks prior to administration of folate-FITC in order to lower serum folate levels to physiologically relevant concentrations. Control rats were also maintained on a folate-deficient diet but not induce...
example 3
Elimination of Endogenous Macrophages
[0049] Evaluation of macrophage independent uptake of the folate-linked imaging agent was accomplished by killing endogenous macrophages with liposomal clodronate. Liposomes were formed by rehydrating a thin film of egg phosphatidylcholine (60 mole %) and cholesterol (40 mole %) in an isotonic clodronate solution (250 mg / ml). Small unilamellar vesicles were then generated by extrusion of the liposomes ten times through a 100 nm polycarbonate membrane using a 10 ml thermobarrel extruder (Lipex Biomembranes, Vancouver, Canada). Unencapsulated clodronate was removed by dialysis through a Spectrapor 300,000 Mr-cutoff cellulose acetate membrane (Spectrum Laboratories, Rancho Domingues, Calif.), and the clodronate concentration in the retained liposomes was determined as described in J Microencapsul. 3(2) 109-14 (1986). Seventeen days following induction of the arthritis and three days prior to administration of the imaging agent (EC20), rats destined...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com