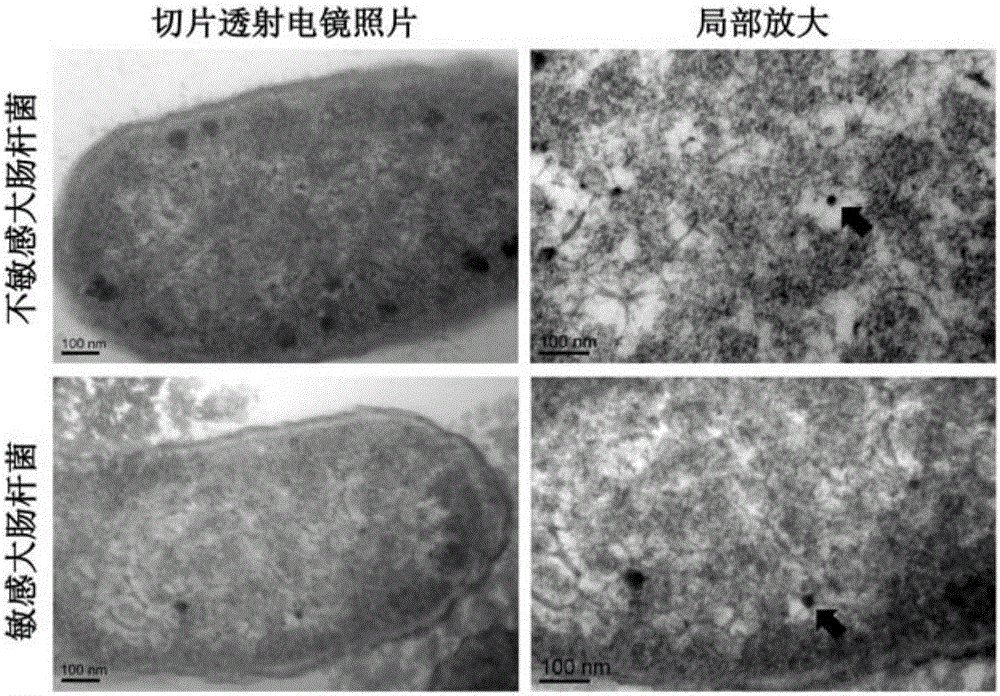
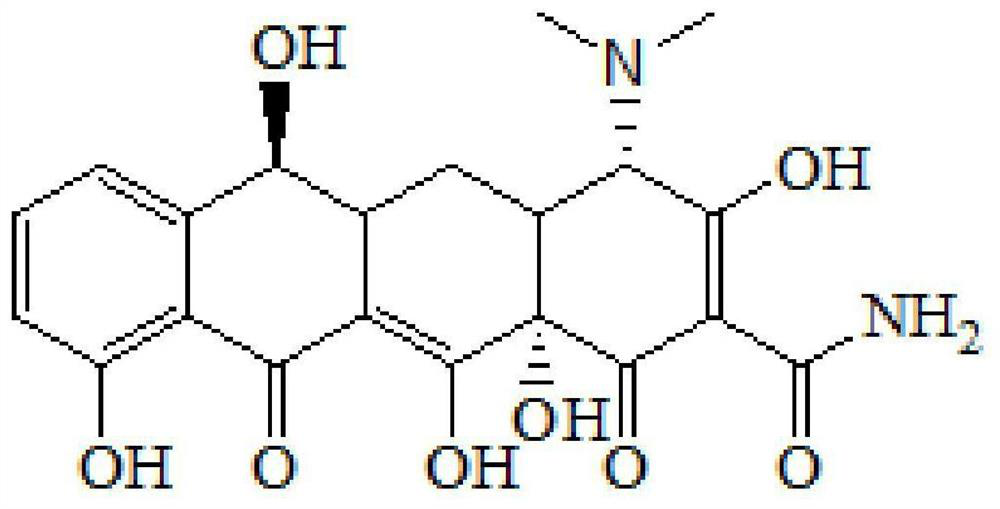
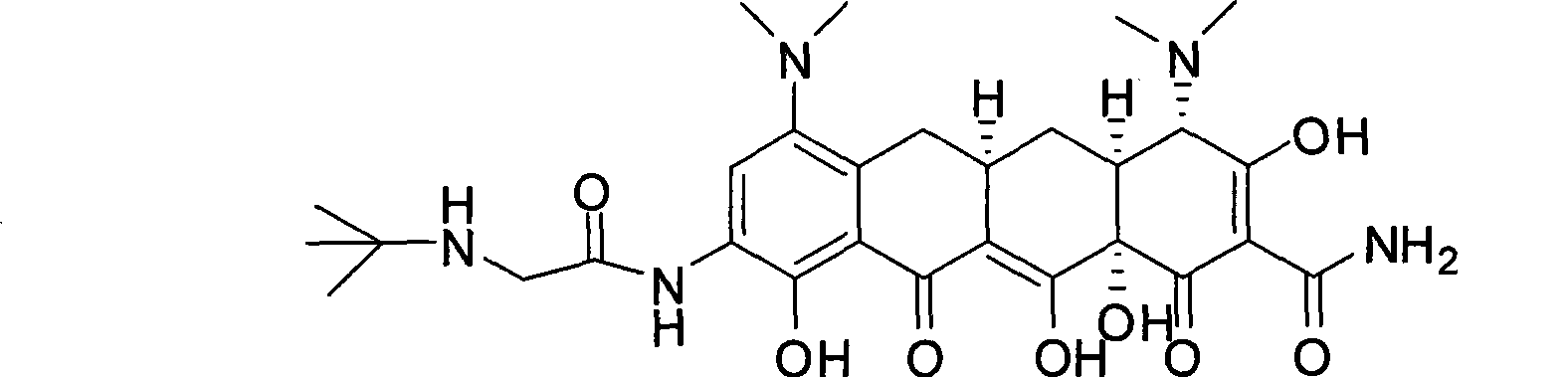
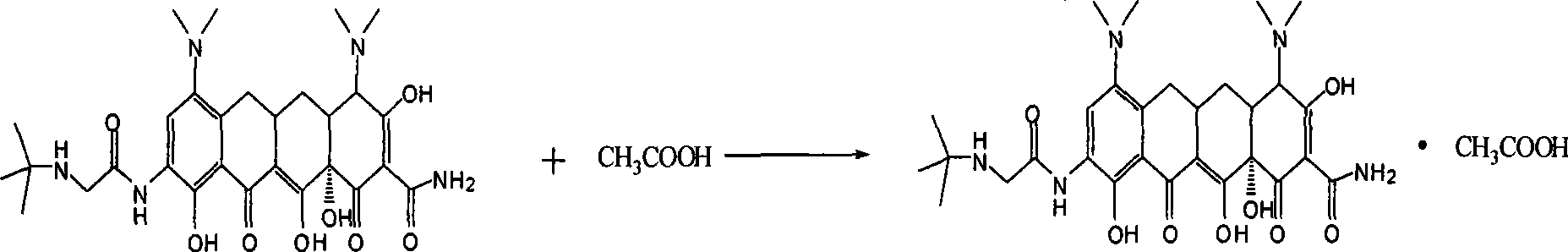
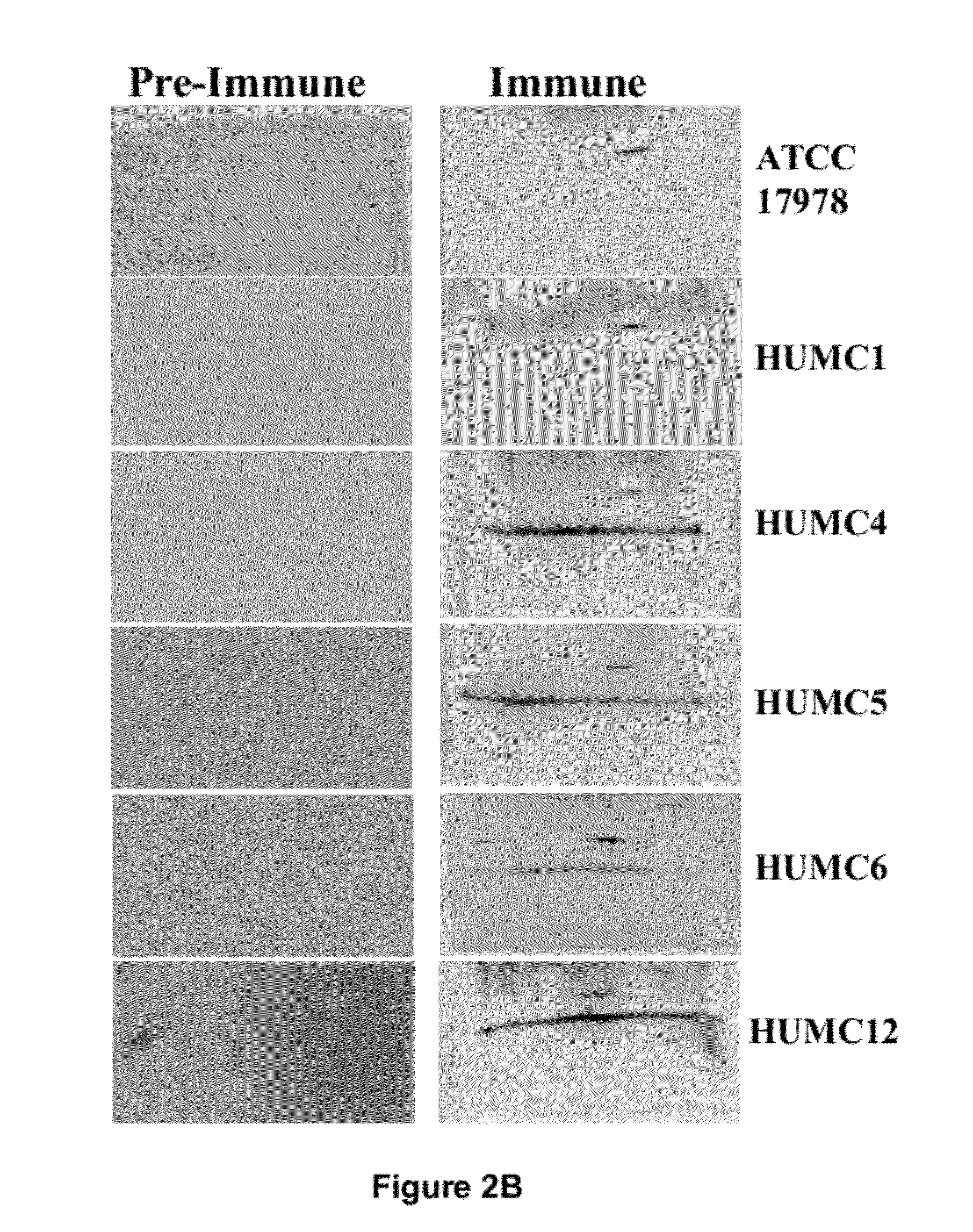
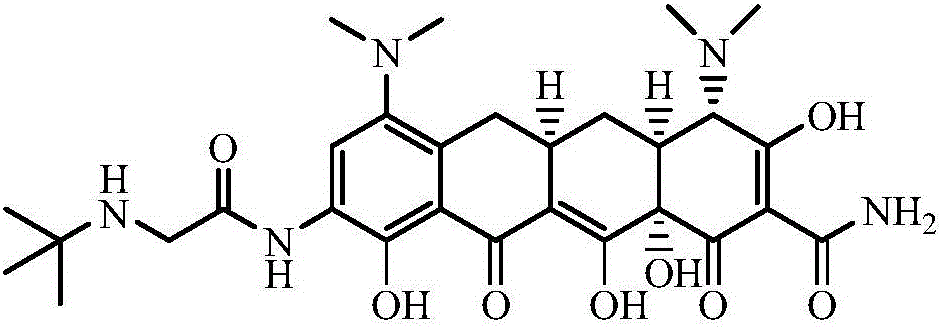
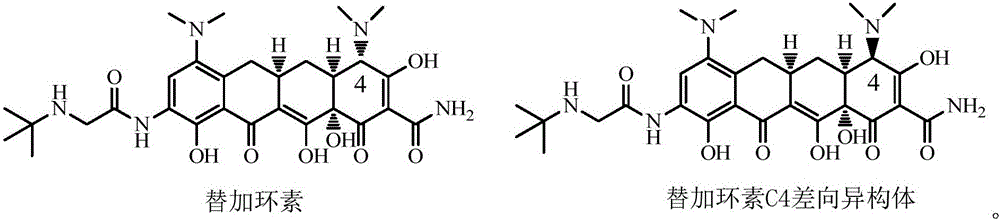
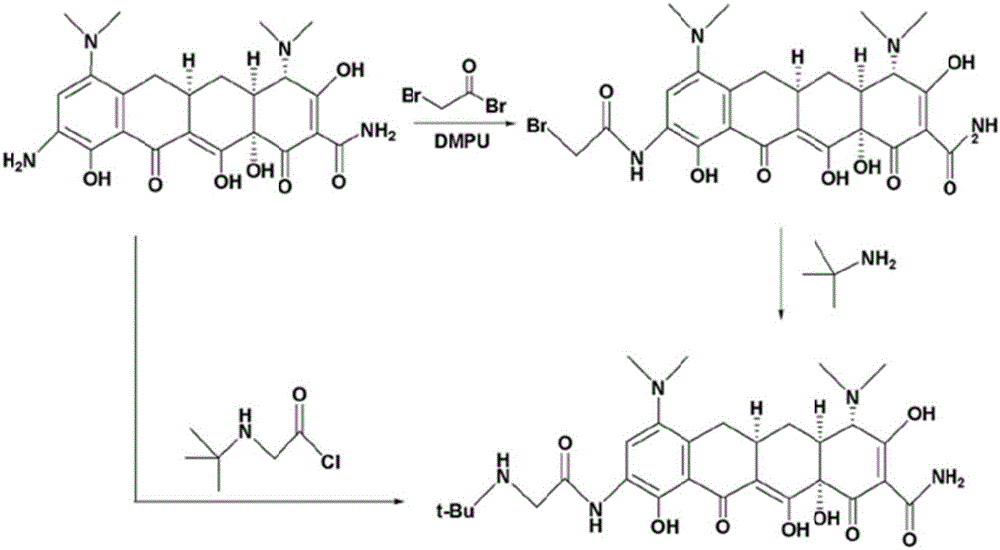
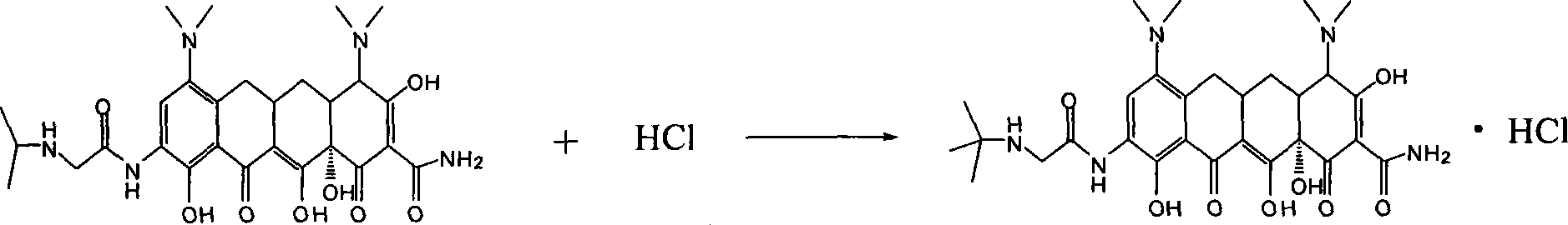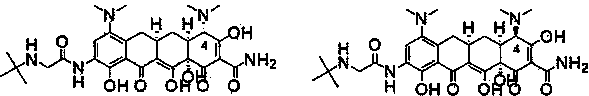Patents
Literature
147 results about "Tigecycline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tigecycline is used to treat certain serious bacterial infections when other antibiotics may not work.
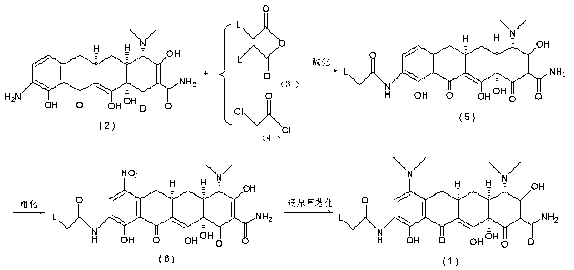
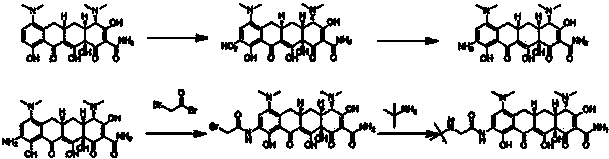
Manufacturing process for tigecycline
Disclosed herein is a manufacturing process for the preparation of tigecycline suitable for intravenous infusion.
Owner:WYETH LLC
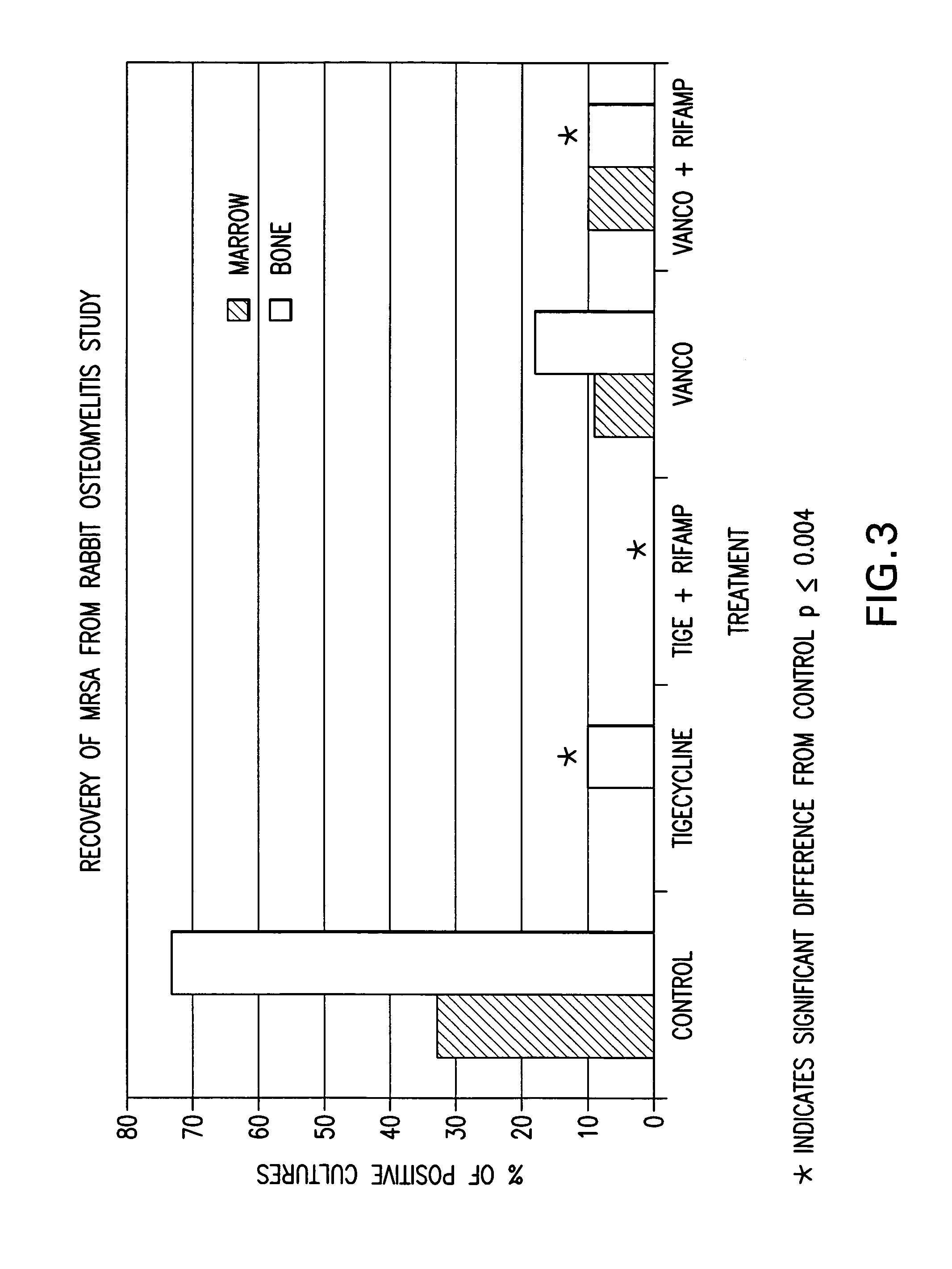
Use of tigecycline, alone, or in combination with rifampin to treat osteomyelitis and/or septic arthritis
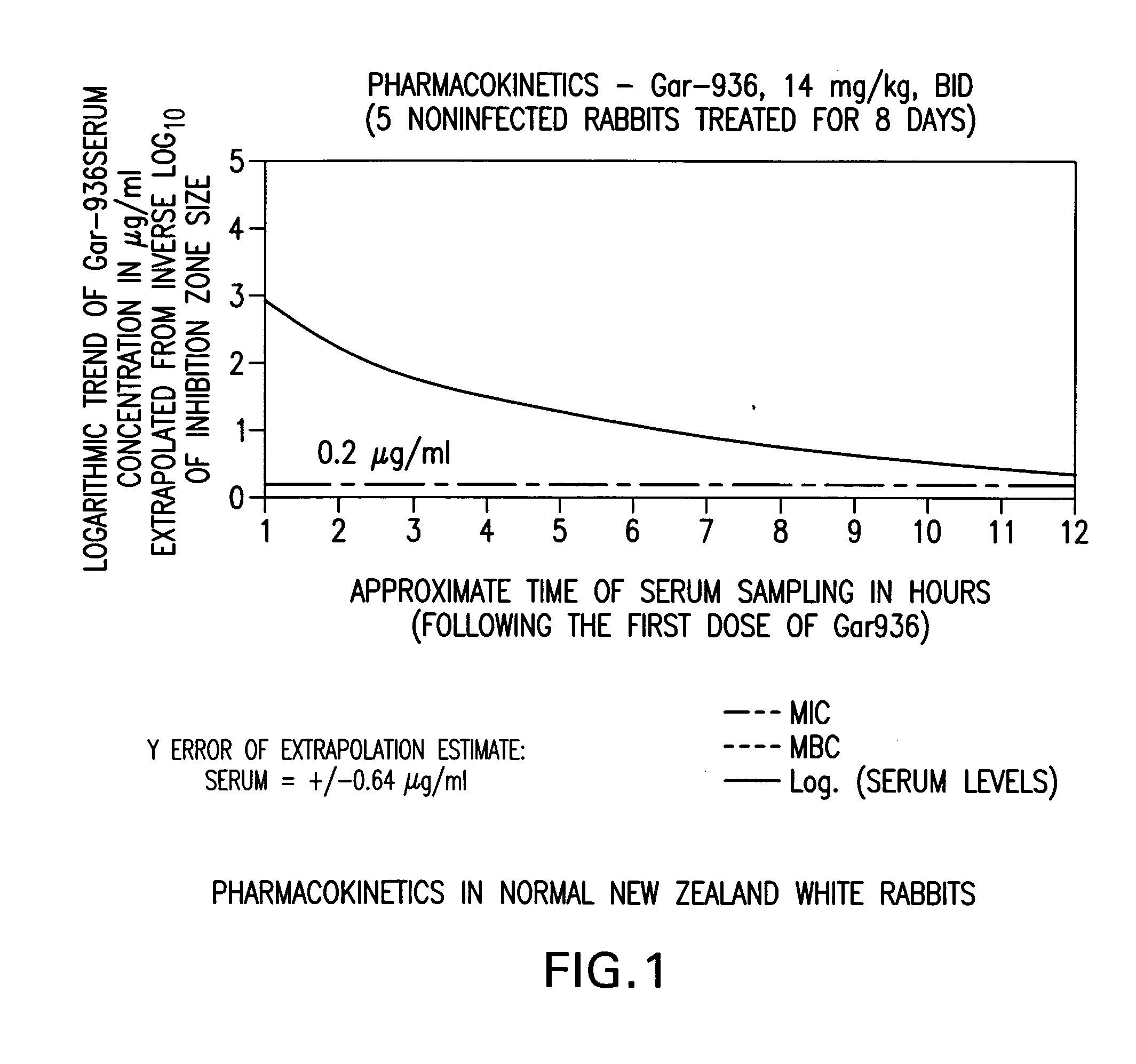
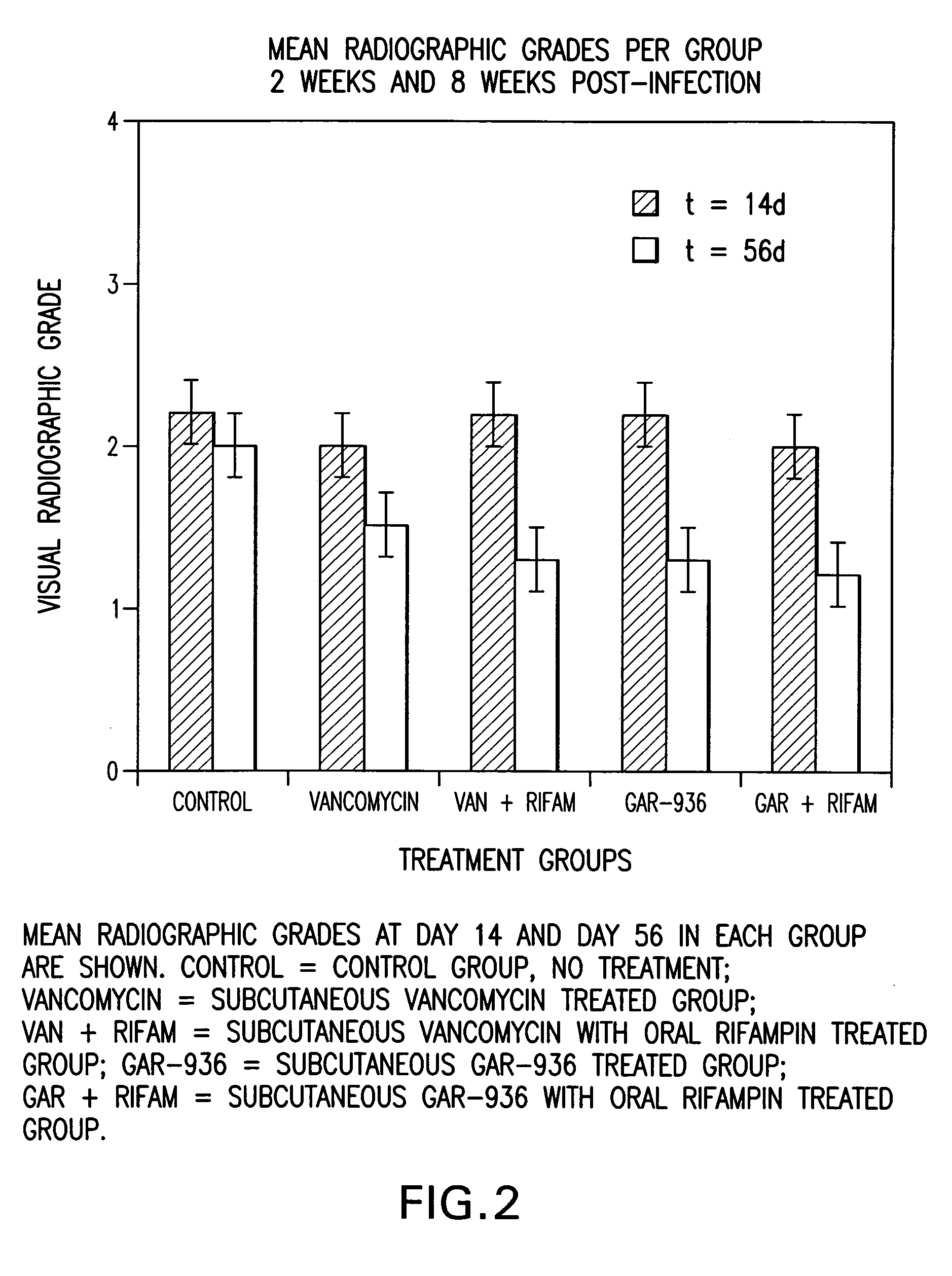
The present invention is directed to a method for treating bone or bone marrow infections, joint infection or infection of the tissues surrounding the joint by administration of the antibiotic tigecycline alone or in combination with a rifamycin antibiotic. In a preferred embodiment the bone or bone marrow infection causes osteomyelitis. In another embodiment the joint infection or infection of the tissues surrounding the joint causes septic arthritis. The invention is also directed to manufacture of a medicament for treatment of bone and / or bone marrow infections, or joint infections and / or infections in tissues surrounding the joint with tigecycline alone or in combination with rifampin.
Owner:WYETH +1
Tigecycline and methods of preparing intermediates
InactiveUS20090099376A1Facilitate large scale synthesisReduce the presence of impuritiesOrganic compound preparationCarboxylic acid amides preparationTigecyclineMedicinal chemistry
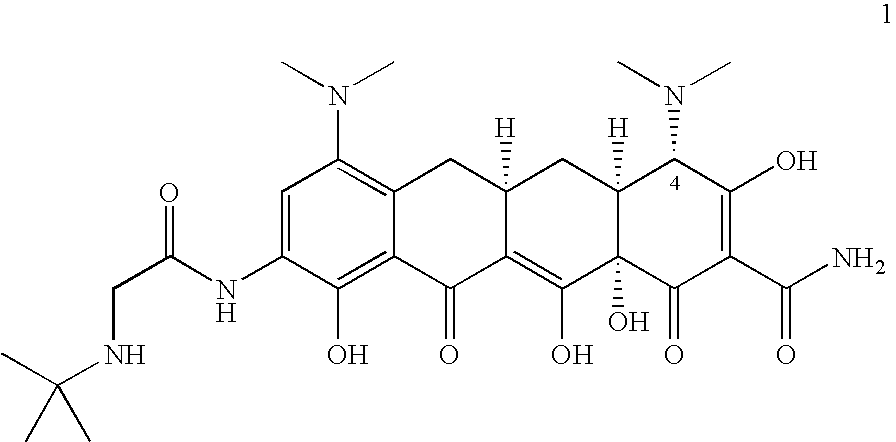
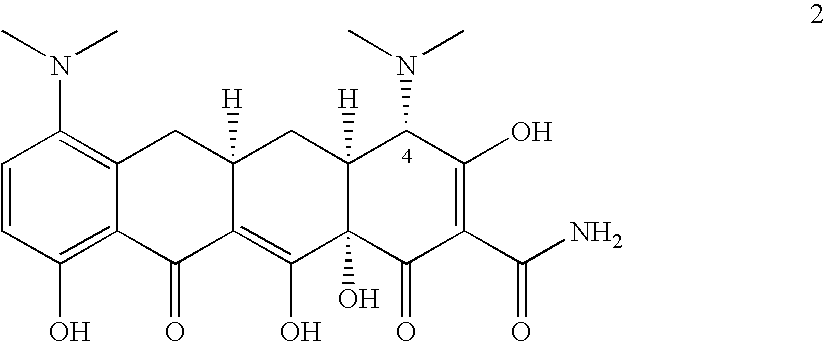
Methods of preparing and purifying 9-nitrominocycline and 9-aminominocycline and salts thereof used in the process of making tigecycline, are disclosed. In one embodiment, the invention is directed to a method of preparing the compound of formula 1or a pharmaceutically acceptable salt thereof, comprising:(a) reacting nitric acid with the compound of formula 2,or a salt thereof, to produce a reaction mixture comprising an intermediate; and(b) further reacting the intermediate to form the compound of formula 1, wherein the intermediate is isolated from the reaction mixture, the method further comprising sparging with an inert gas prior to step (a).
Owner:WYETH
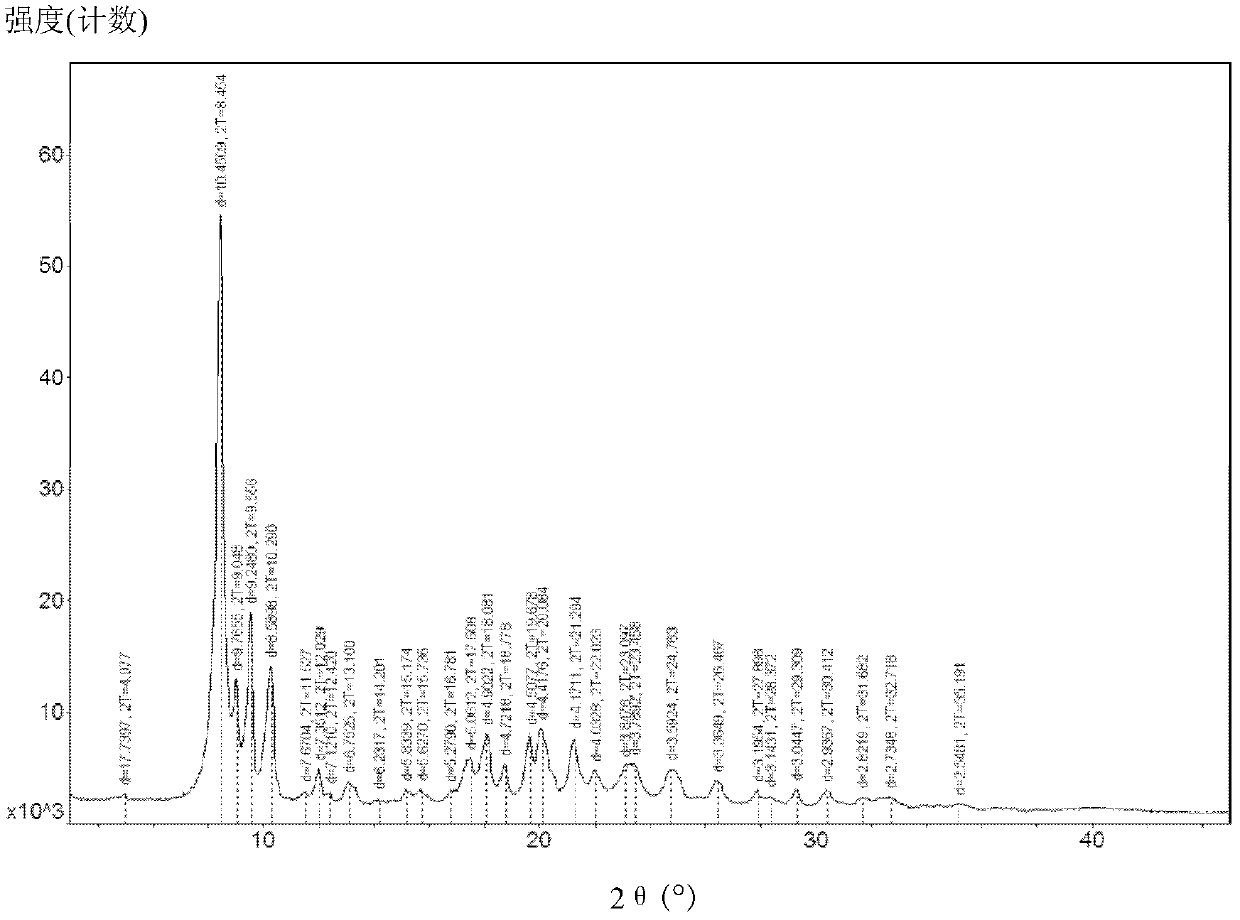
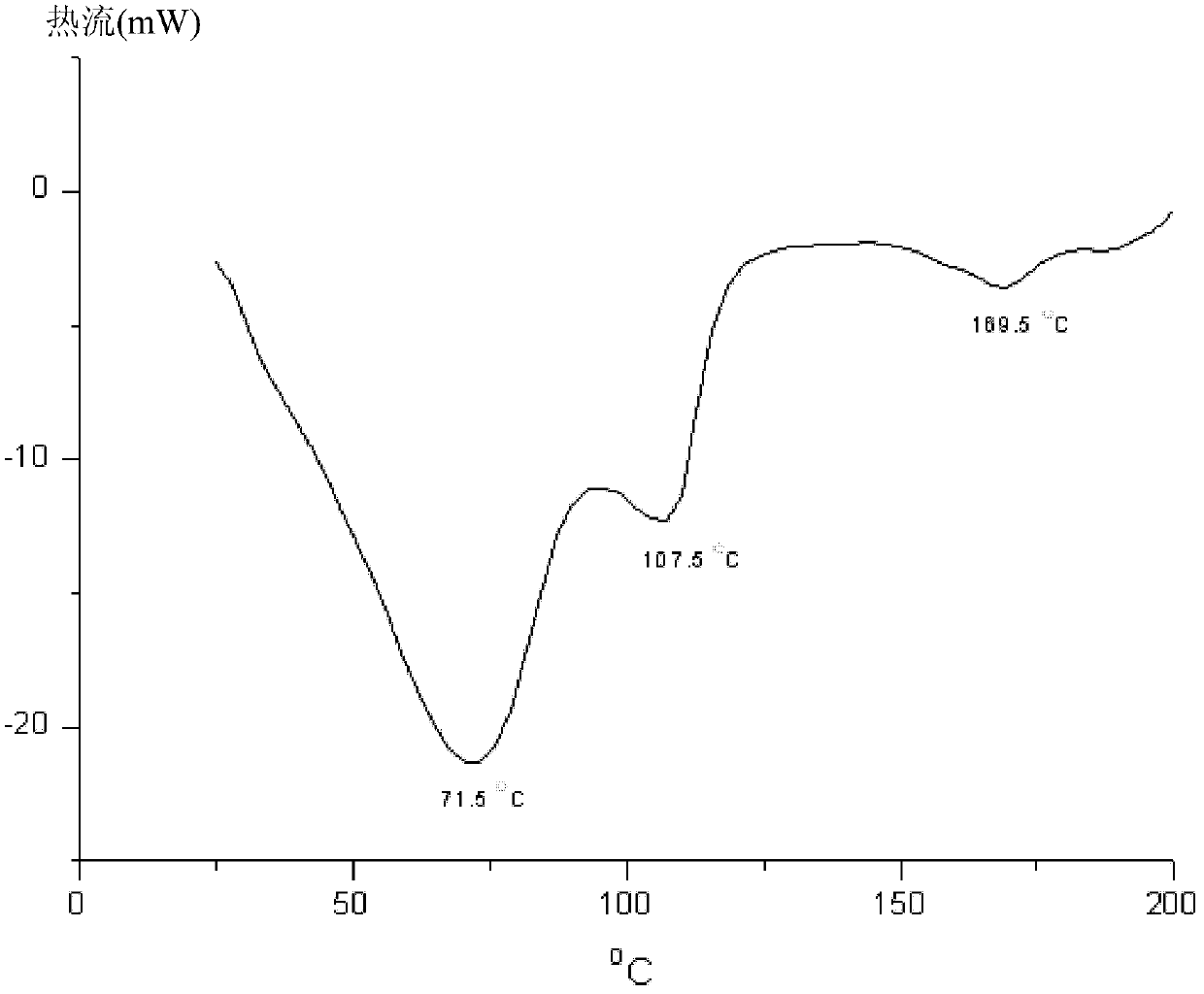
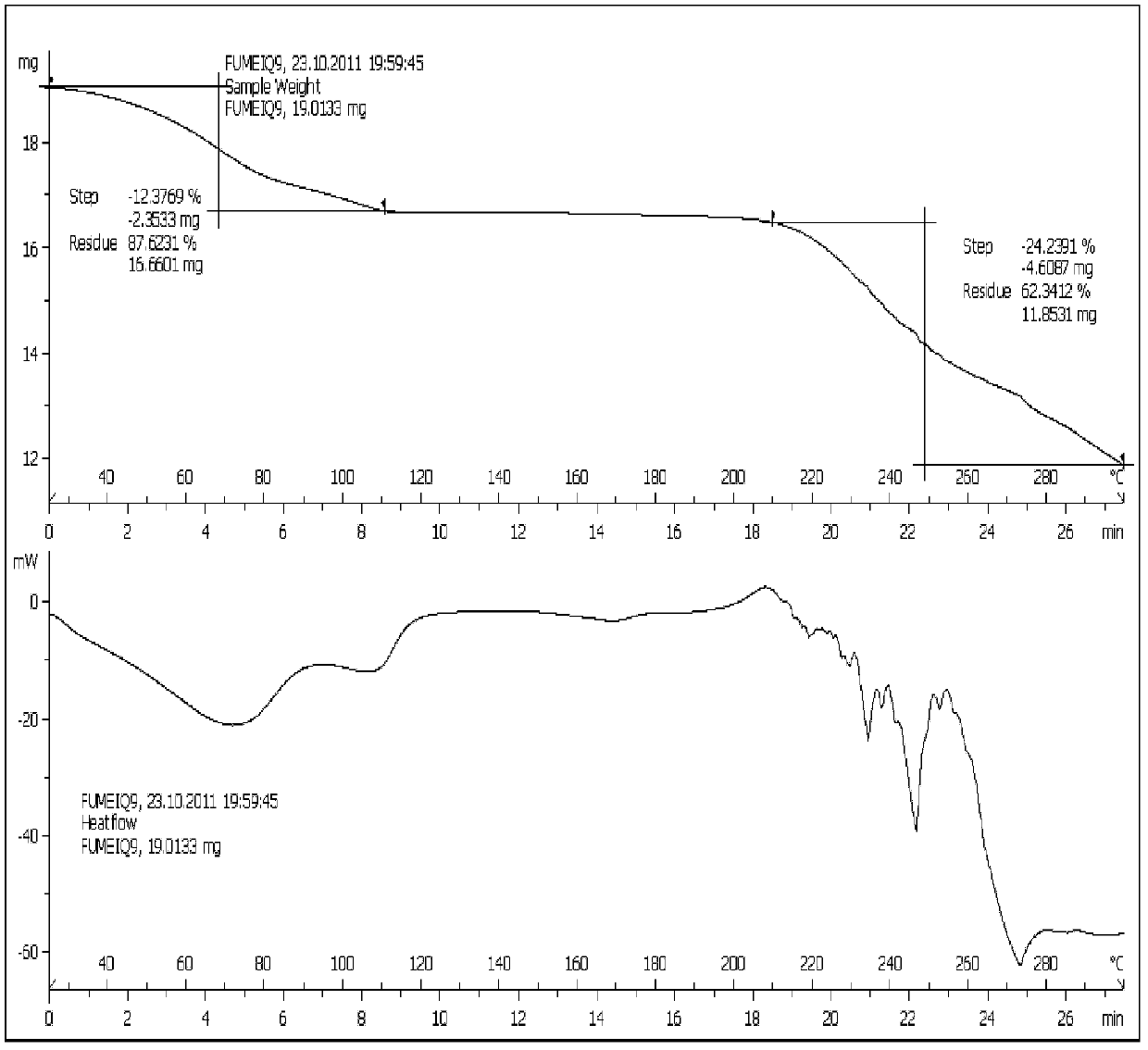
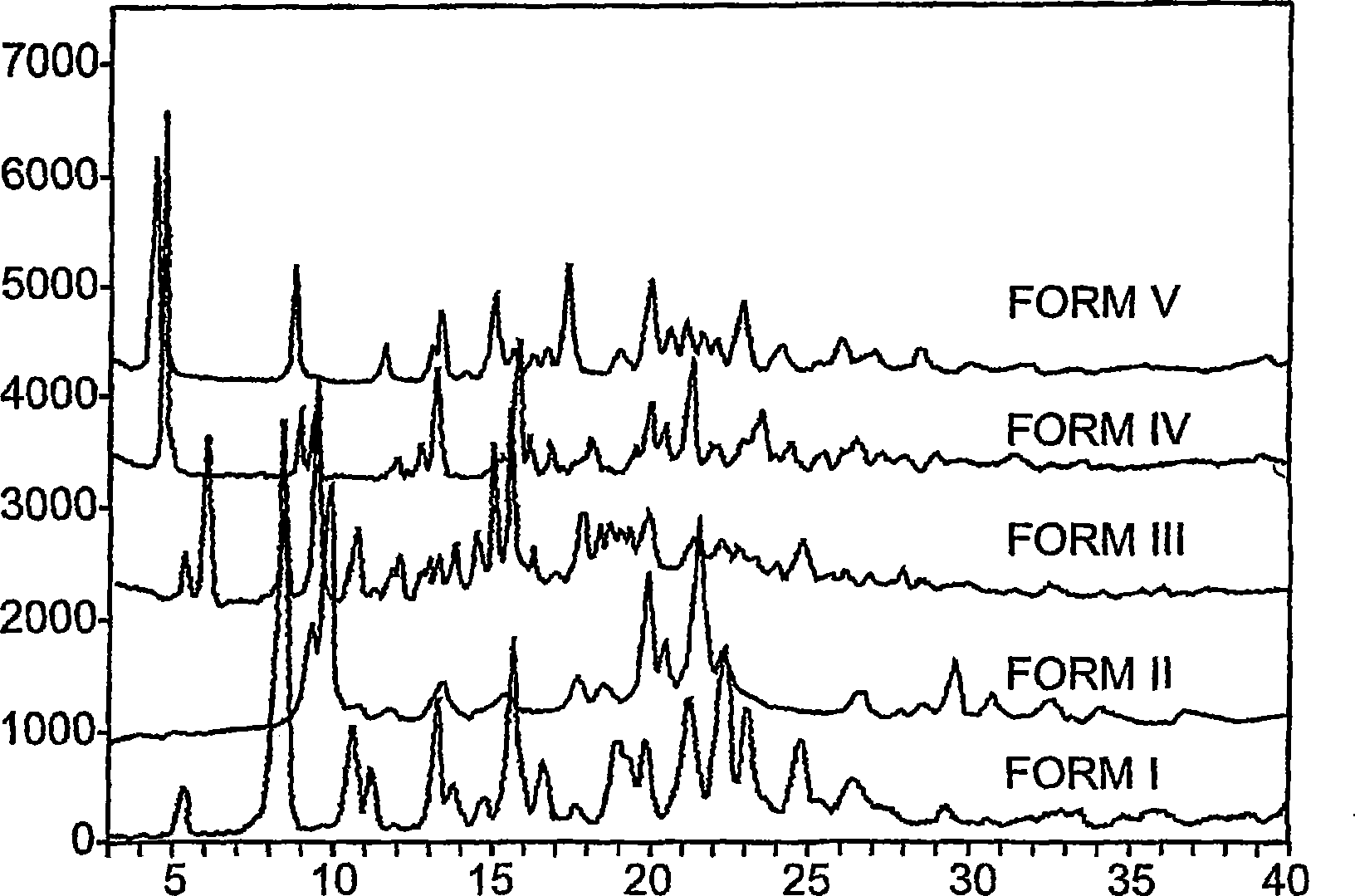
New tigecycline crystal form and preparation method thereof
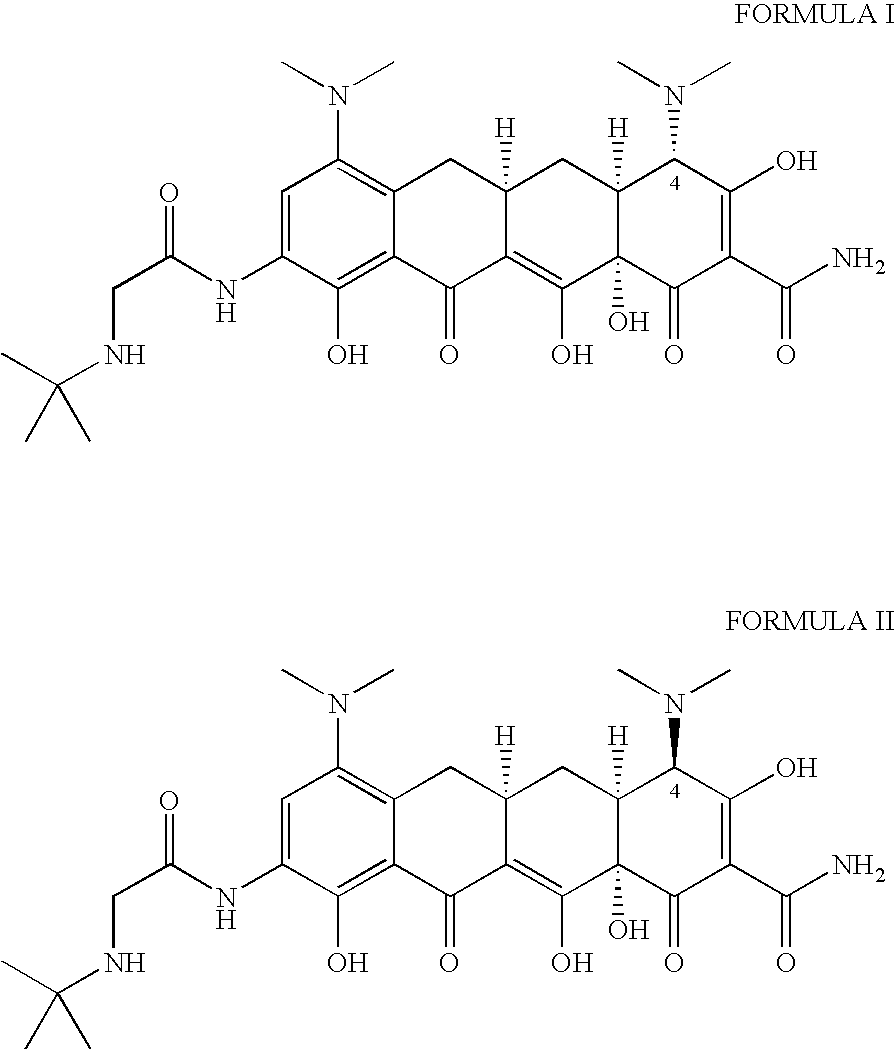
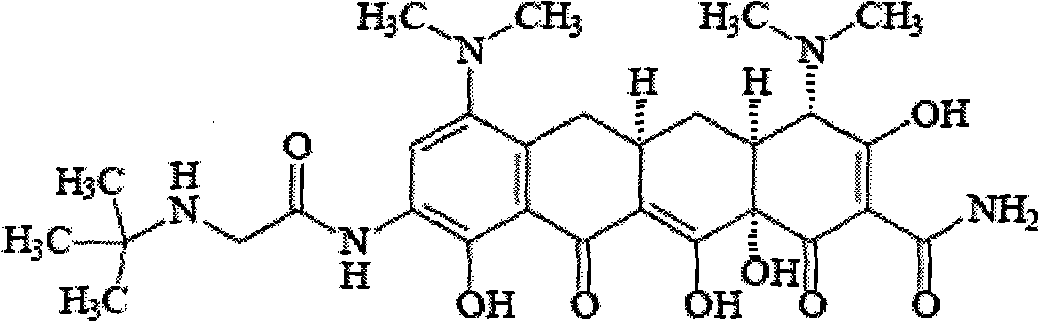
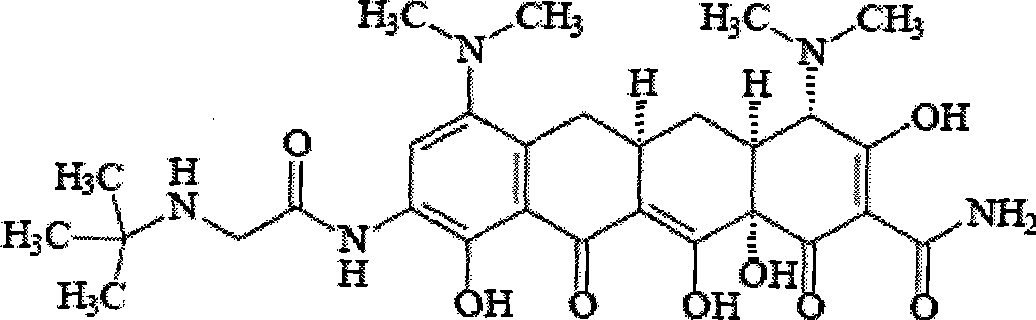
The invention relates to a new tigecycline crystal form and a preparation method thereof. The new tigecycline crystal form is represented by a formula (I) and is a new crystal form of (4S, 4aS, 5aR, 12aS)-9-(2-(tertiary butyl amino) acetamido)-4,7-bis(dimethyl amino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacene formamide (tigecycline). The new tigecycline crystal form represented by the formula (I) is suitable for the preparation of freeze-dried powder.
Owner:宫宁瑞
Tigecycline freeze-dried injection
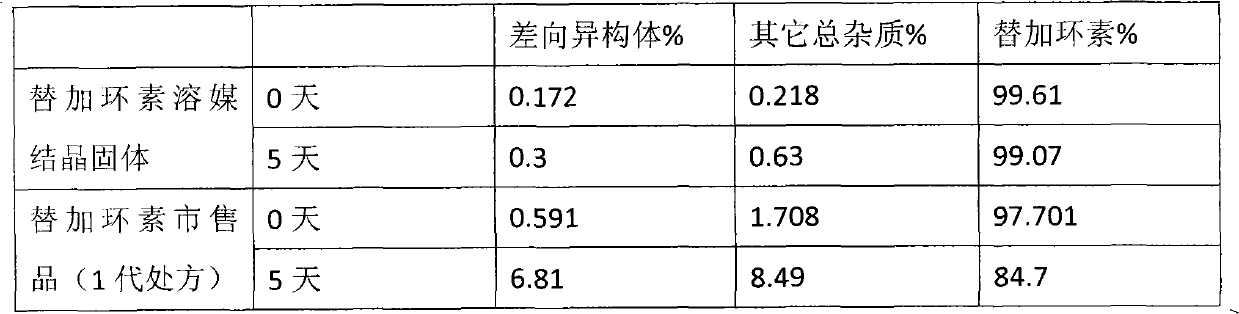
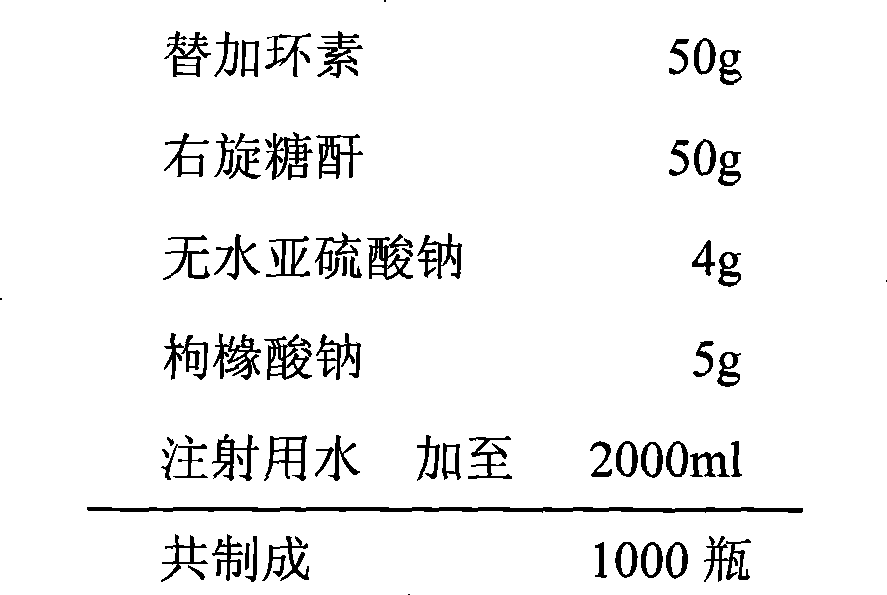
ActiveCN101401812AImprove stabilityLow content of related substancesAntibacterial agentsPowder deliverySulfite saltFreeze-drying
The invention relates to a tigecycline freeze-dried powder injection, which consists of tigecycline, dextran, sodium sulfite and sodium citrate according to the following weight proportion: 10 portions of the tigecycline, 10 to 90 portions of the dextran, 0.1 to 3 portions of anhydrous sodium sulfite, and 0.1 to 3 portions of the sodium citrate. The pH value of the tigecycline freeze-dried powder injection is between 7.0 and 9.0. The preparing process comprises the following steps: firstly, dissolving the dextran by 80 percent of water for injection; secondly, adding the sodium citrate and the anhydrous sodium sulfate into the mixture after cooling; thirdly, adding the tigecycline into the mixture after dissolving and evenly stirring the sodium citrate and the anhydrous sodium sulfate, and mixing the mixture evenly; and fourthly, regulating the pH to between 7.0 and 9.0, adding a needle activated carbon into the mixture, stirring and adsorbing the mixture, removing the activated carbon from the mixture, and fixing the capacity. The solution is filtered through two microporous membranes and then are filled in a cillin bottle, and the tigecycline freeze-dried powder injection is obtained through semi-stoppering, spanning, freeze-drying, introducing nitrogen, performing tamponade, taking out the injection from a box, rolling a mouth, passing the quality inspection, and packaging.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Synthetic method for high-purity tigecycline
ActiveCN102391148AAvoid degradationShort synthetic routeOrganic compound preparationCarboxylic acid amides preparationGlycineTigecycline
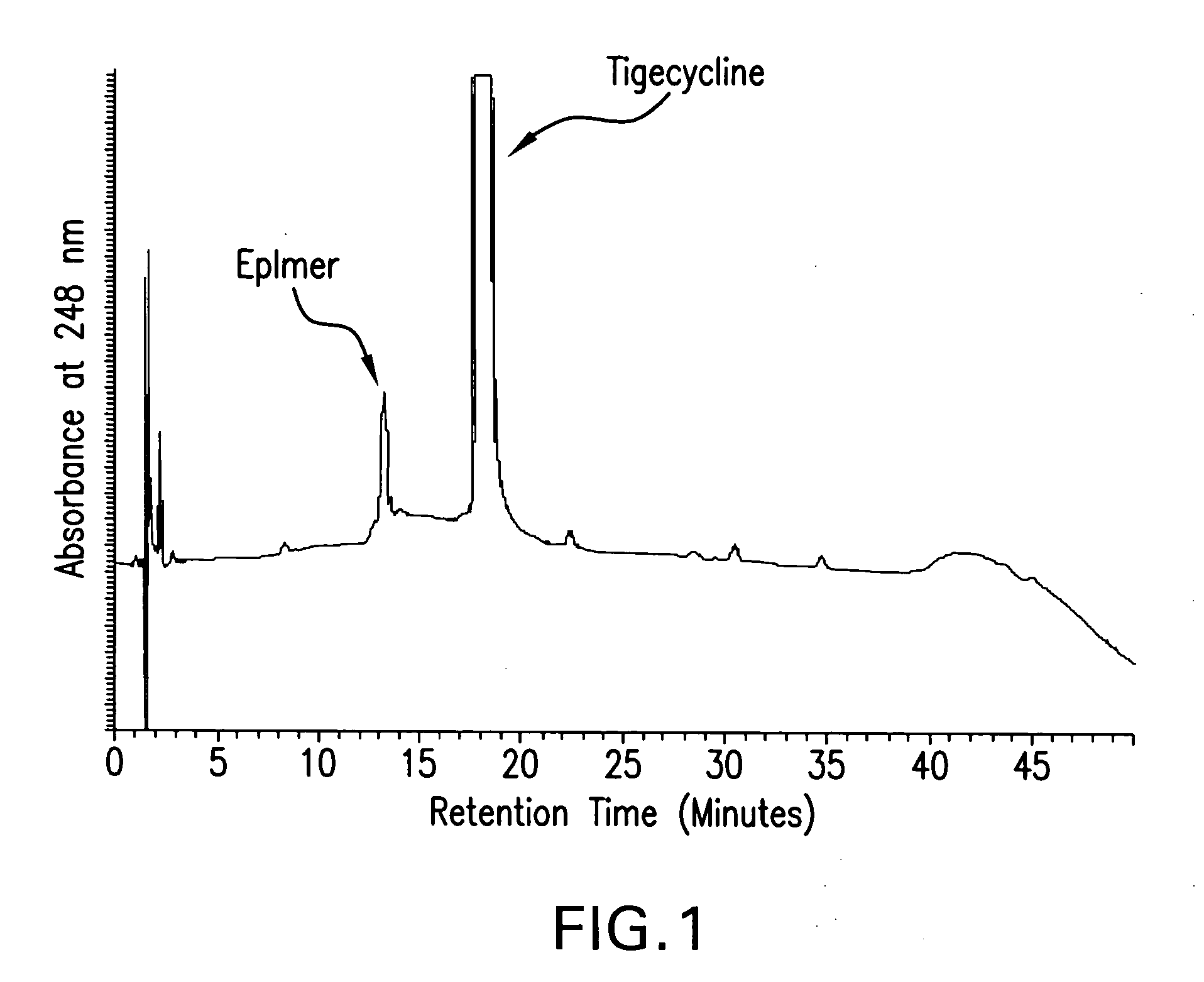
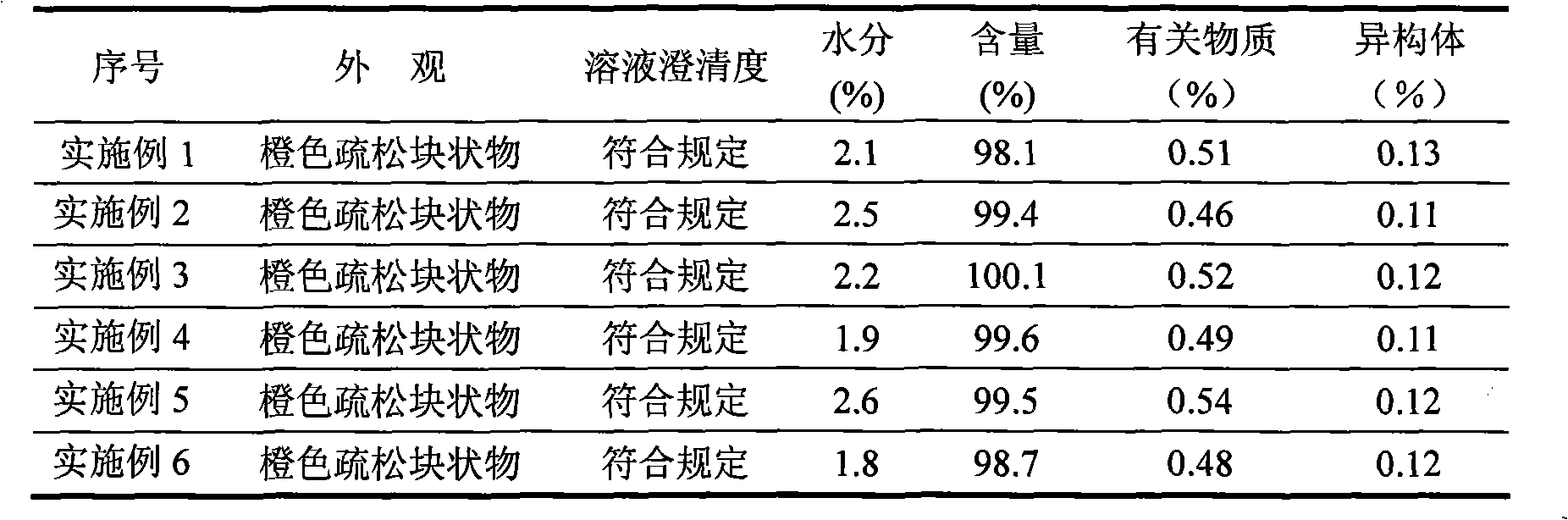
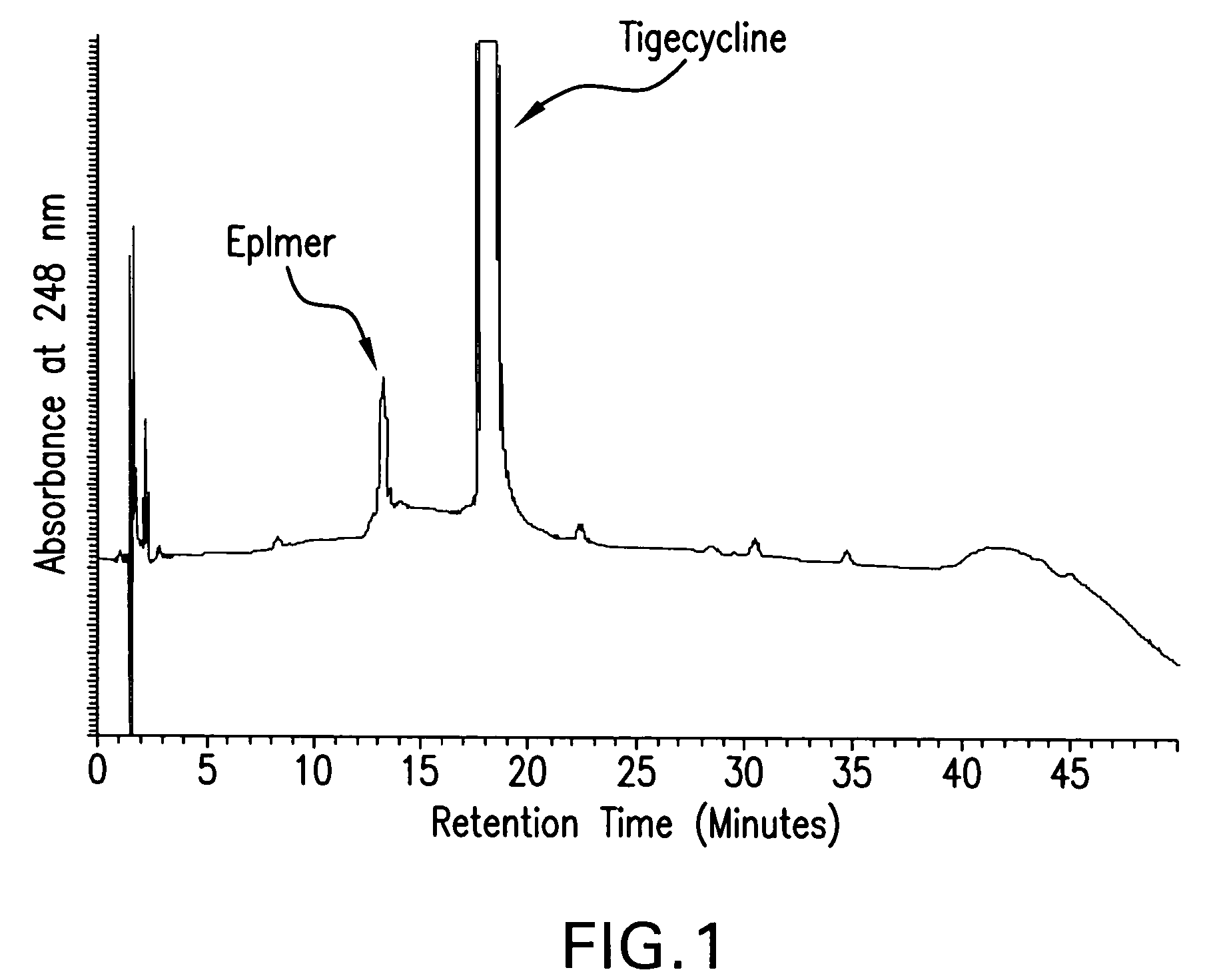
The invention relates to a novel preparation method for antibioticdrug tigecycline. 9-amino minocycline and N-tert-butyl group glycine are taken as starting materials. The novel preparation method is characterized in that the N-tert-butyl group glycine is dissolved in indifferent solvent, under the existence of an acidic acceptor and an amino acid condensating agent, reaction with 9-amino minocycline is carried out along the route of amino acid condensation for 3 hours to 10 hours, and then an indifferent solvent is cooled to a room temperature. Tigecycline is obtained through acidification, neutralization, extraction, drying, concentration and refining. In the preparation method disclosed by the invention, the operation is simplified, the purity of the obtained product reaches more than 99.5 percent, individual impurities are controlled to be lower than 0.1 percent, and an epimer is controlled to be lower than 0.5 percent; and the yield is high, the stability of the product is good, and the preparation method is suitable for industrial production.
Owner:NANJING HAIRUN PHARM CO LTD
Tigecycline without crystal habit and method of preparing the same
InactiveCN101367747AHigh antibacterial activityCarboxylic acid amide separation/purificationTigecyclineCrystal habit
The present invention provides a no-crystalline state tigecycline and a preparation method of the tigecycline. The present invention has obvious bacteriostasis activity.
Owner:SHANGHAI LAIYI BIOMEDICAL RES & DEV CENT +1
Tigecycline-containing sterile packaged preparation for injection
ActiveCN101919816AFast dissolutionImprove stabilityAntibacterial agentsPowder deliveryTigecyclineSolvent
The invention belongs to the field of pharmaceuticals and relates to a tigecycline-containing sterile packaged preparation for injection. The sterile powder of tigecycline is prepared by a solvent crystallization method. Each preparation contains 10 to 500mg of sterile powder of tigecycline or sterile powder of tigecycline salt.
Owner:SHIJIANGZHUANG ZHIHENG PHARMACY TECH CO LTD
Tigecycline freeze-dried injection
ActiveCN101401812BImprove stabilityLow content of related substancesAntibacterial agentsPowder deliverySulfite saltFreeze-drying
The invention relates to a tigecycline freeze-dried powder injection, which consists of tigecycline, dextran, sodium sulfite and sodium citrate according to the following weight proportion: 10 portions of the tigecycline, 10 to 90 portions of the dextran, 0.1 to 3 portions of anhydrous sodium sulfite, and 0.1 to 3 portions of the sodium citrate. The pH value of the tigecycline freeze-dried powderinjection is between 7.0 and 9.0. The preparing process comprises the following steps: firstly, dissolving the dextran by 80 percent of water for injection; secondly, adding the sodium citrate and the anhydrous sodium sulfate into the mixture after cooling; thirdly, adding the tigecycline into the mixture after dissolving and evenly stirring the sodium citrate and the anhydrous sodium sulfate, and mixing the mixture evenly; and fourthly, regulating the pH to between 7.0 and 9.0, adding a needle activated carbon into the mixture, stirring and adsorbing the mixture, removing the activated carbon from the mixture, and fixing the capacity. The solution is filtered through two microporous membranes and then are filled in a cillin bottle, and the tigecycline freeze-dried powder injection is obtained through semi-stoppering, spanning, freeze-drying, introducing nitrogen, performing tamponade, taking out the injection from a box, rolling a mouth, passing the quality inspection, and packaging.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
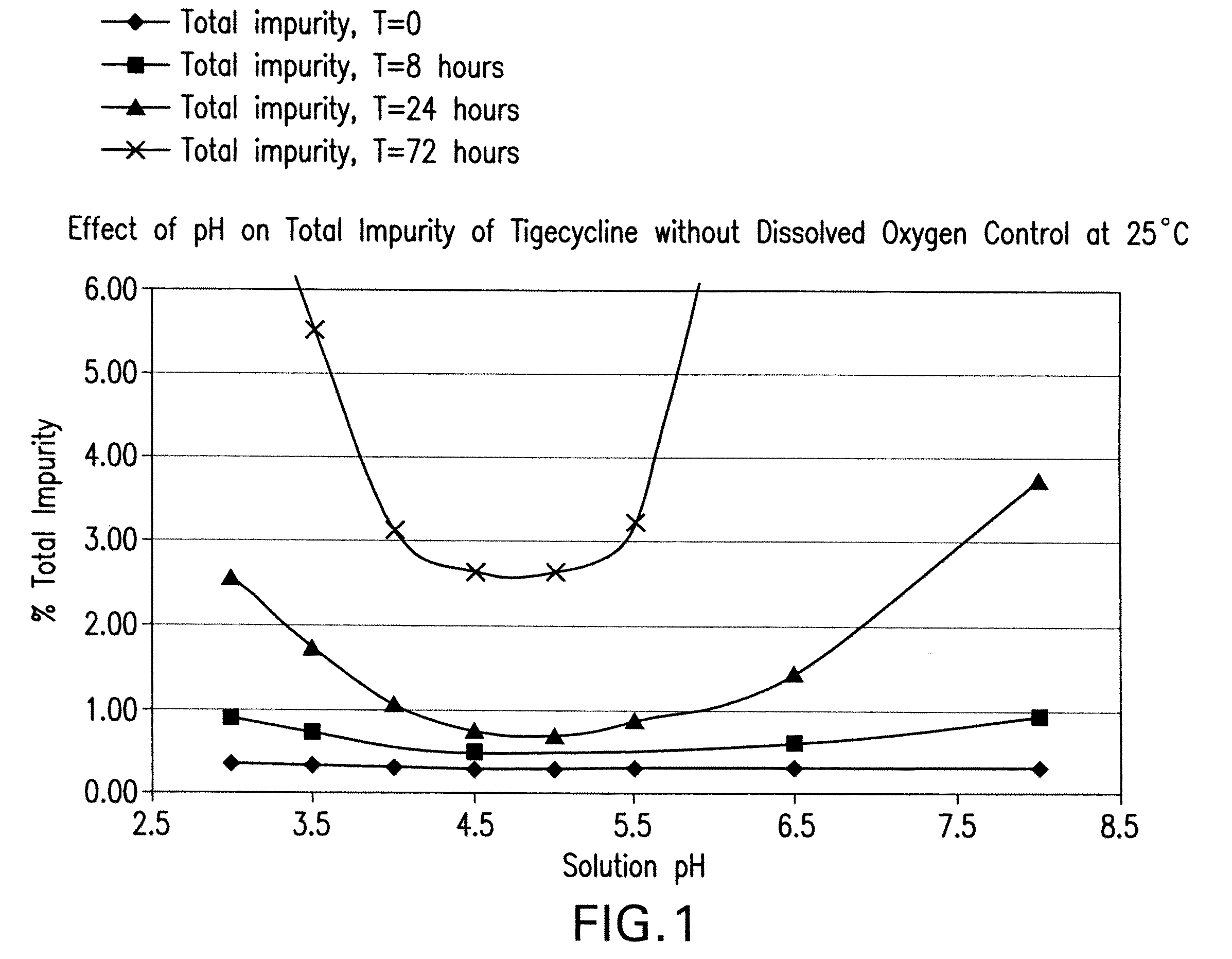
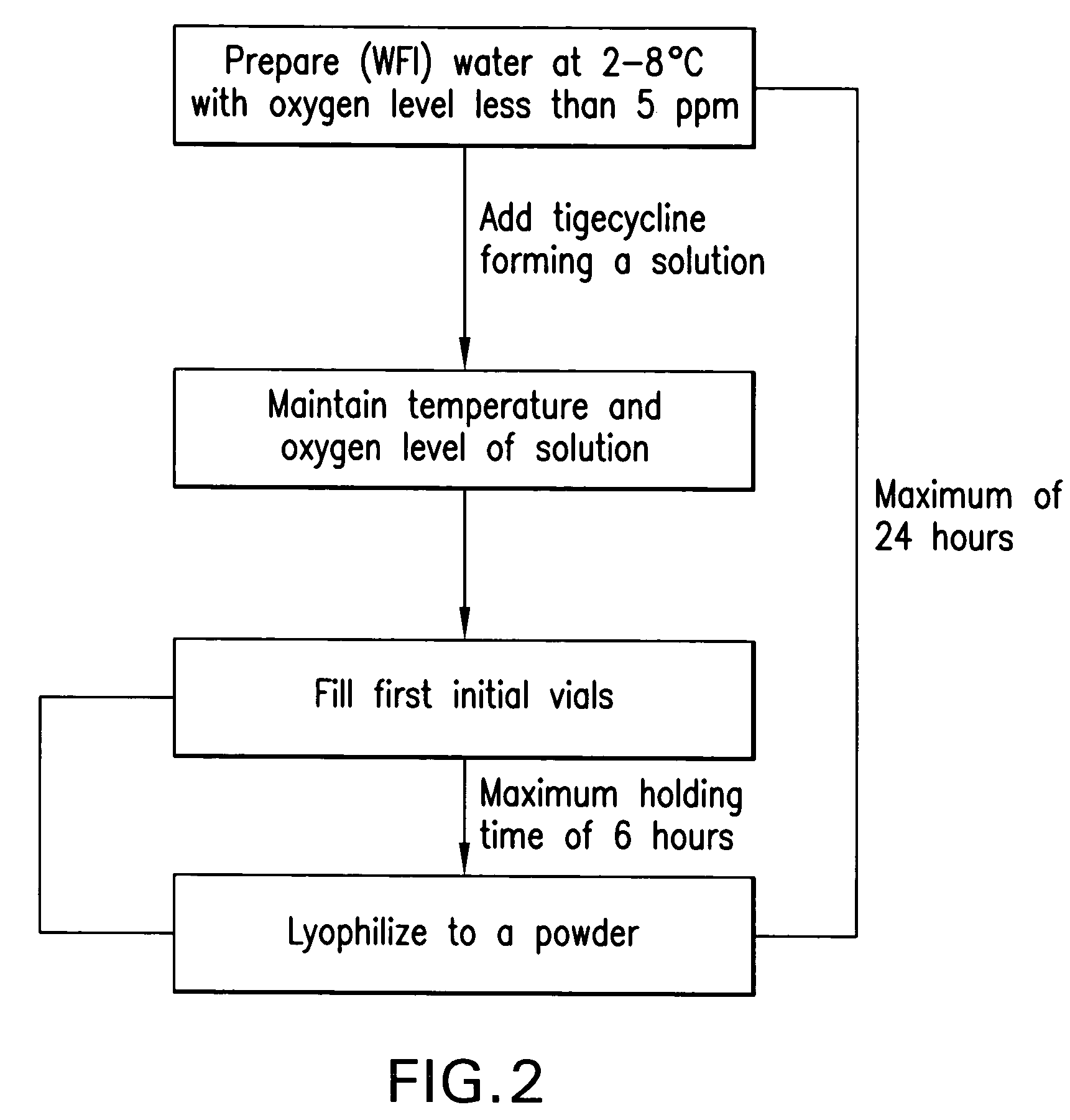
Prepnaring method for freeze dried tigecycline powder for injection
InactiveCN1985835ASolve the problem of oxidative degradationClinical drug safetyAntibacterial agentsPowder deliveryTigecyclineFreeze-drying
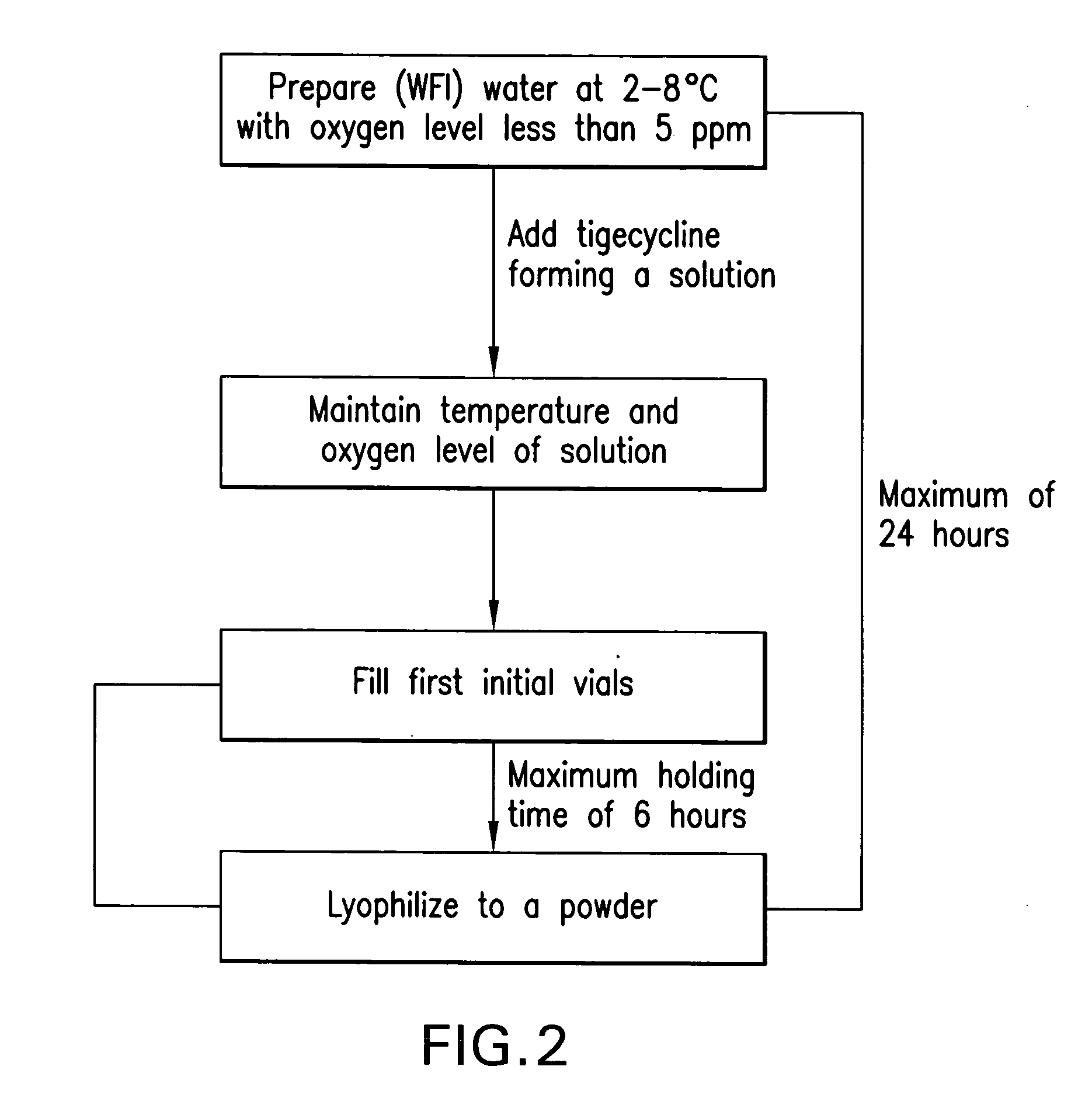
The present invention relates to preparation process of freeze dried tigecycline powder for injection. The preparation process includes the following steps: introducing clean treated air into water for injection at 5-30deg.c to replace its oxygen, adding tigecycline through stirring for dissolving, adding active carbon for injection in 0.1 g / 100ml while further introducing clean air and stirring for 15-30 min, coarse filtering to eliminate active carbon, filtering with 0.45 micron microporous membrane, introducing clean air into the filtrate, filtering with 0.22 micron microporous membrane, packing in 5-15 ml size antibiotic ampoule, pre-freezing for 3-6 hr, freeze drying for 24-48 hr, covering, and rolling aluminum cap. The present invention has raised clinical safety.
Owner:合肥信风科技开发有限公司
Nutritional seed-soaking fluid for crop seeds
InactiveCN105875671AGive full play to the bactericidal nutritional effectImprove efficiencyBiocidePlant growth regulatorsDiseaseCyclohexanone
The invention relates to a nutritional seed-soaking fluid for crop seeds. The nutritional seed-soaking fluid is prepared from the following components by weight: 1500g of purified water, 10g of pollen pini, 10g of black tea powder, 10g of chelated zinc, 20g of calcium hypochlorite, 10g of cyclohexanone, 5g of flos lonicera extract, 3g of tigecycline, 5g of selenious yeast powder, 10g of yellow wine, 5g of zanthoxylum oil, 3g of tobramycin, 5g of 25% tebuconazole wettable powder, 4g of cefradine capsules, 5g of anthocyanin from purple sweet potato, 30g of linden bark powder, 10g of radix notoginseng powder, 10g of cortex dictamni extract, and 15g of compound sodium nitrophenolate. The nutritional seed-soaking fluid disclosed by the invention is prepared by heating purified water to 50 to 65 DEG C, then adding other remaining components, and continuously stirring for about 10 minutes. When seeds are soaked with the fluid disclosed by the invention, the disease resistance of the seeds can be improved, the stress resistance of seedlings is improved, the seed germination is accelerated, and the purposes of disinfection and sterilization are achieved.
Owner:CHAOHU XINYU BREEDING FARMER PROFESSIONAL COOP
Substance conformation of tigecycline and method for preparing same
InactiveCN101134733ASolve the problem of oxidative degradationSmall toxicityCarboxylic acid amide separation/purificationOrganic solventFreeze-drying
The present invention relates to the matter form and preparation process of tigecycline. The amorphous tigecycline is prepared through the following steps: taking water for injection at proper temperature and introducing clean air to replace oxygen dissolved in water, adding tigecycline through slight stirring for dissolving, adding proper amount of active carbon for injection while introducing clean air, filtering to eliminate active carbon, introducing clean air to the filtrate, filtering in microporous filter membrane, pre-cooling, and freeze drying. Thus prepared amorphous tigecycline contains no organic solvent and no organic solvent caused side effect.
Owner:合肥信风科技开发有限公司
Preparation method of tigecycline composition
InactiveCN102641249AQuality improvementLow in proteinAntibacterial agentsPowder deliveryPenicillinFiltration
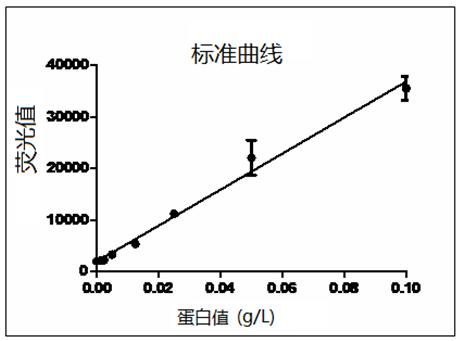
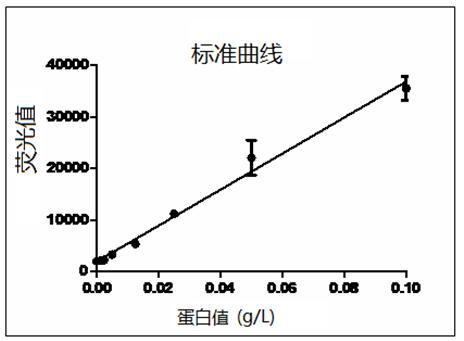
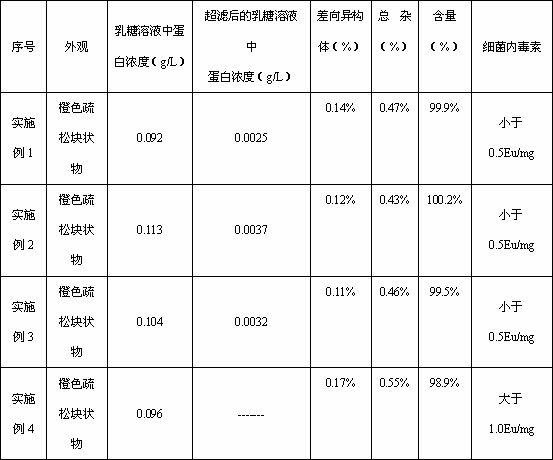
The invention relates to a preparation method of tigecycline composition, which comprises enabling lactose to be dissolved by using 80% water for injection, conducting ultrafiltration treatment on lactose solution, collecting ultrafiltrate, adding tigecycline after the solution is cooled, evenly mixing, adjusting the potential of hydrogen (pH) value to 4.0-6.0, adding activated carbon for a needle for stirring, adsorption and decarburization, and conducting constant volume; enabling the solution to be filled in a penicillin bottle after filtration, conducting semi-plugging, disc loading, freeze-drying, nitrogen communicating, plug pressing, outbox and cover rolling, packaging after quality testing is qualified, and then obtaining the tigecycline composition. The protein concentration of the composition obtaining through the preparation method is smaller than 0.005g / L, untoward effects caused by the fact that lactose is added in the products are greatly reduced, the preparation method is simple and convenient in operation and low in cost, and prepared products are good in quality and easy in industrial production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
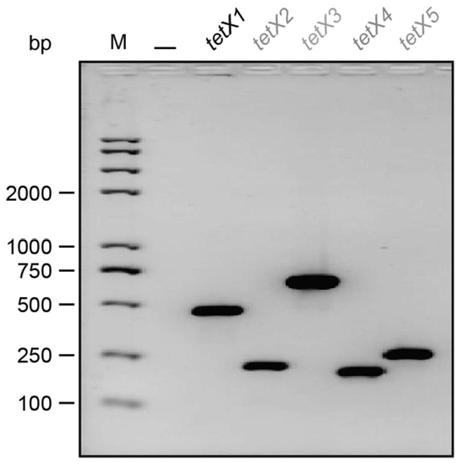
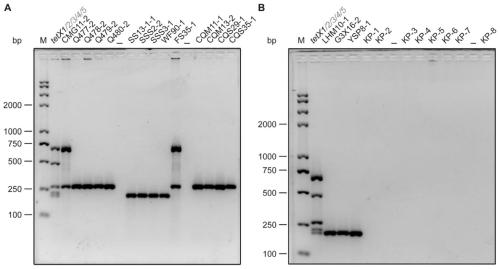
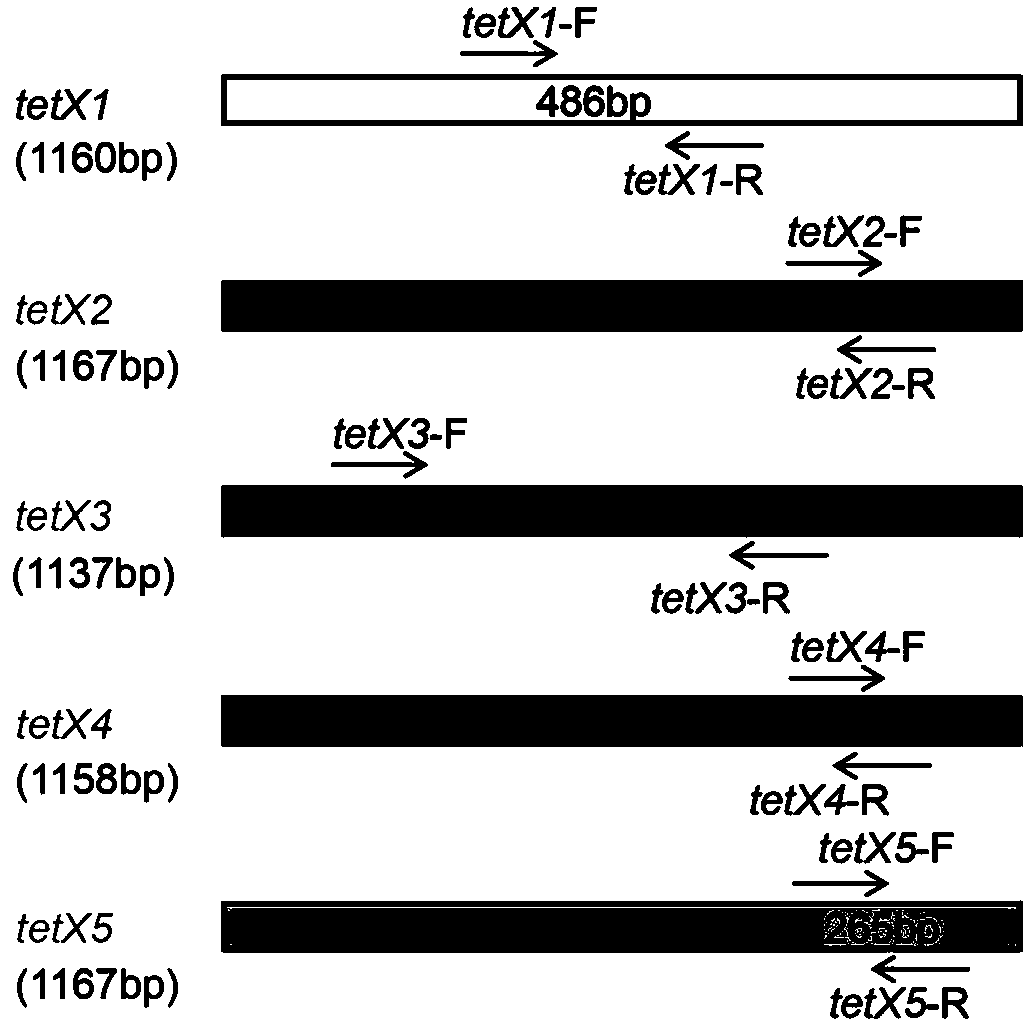
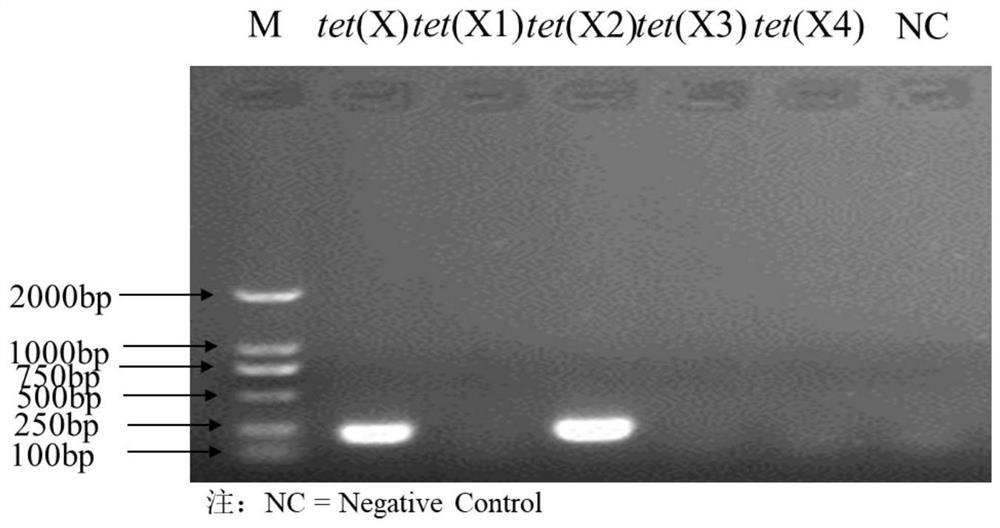
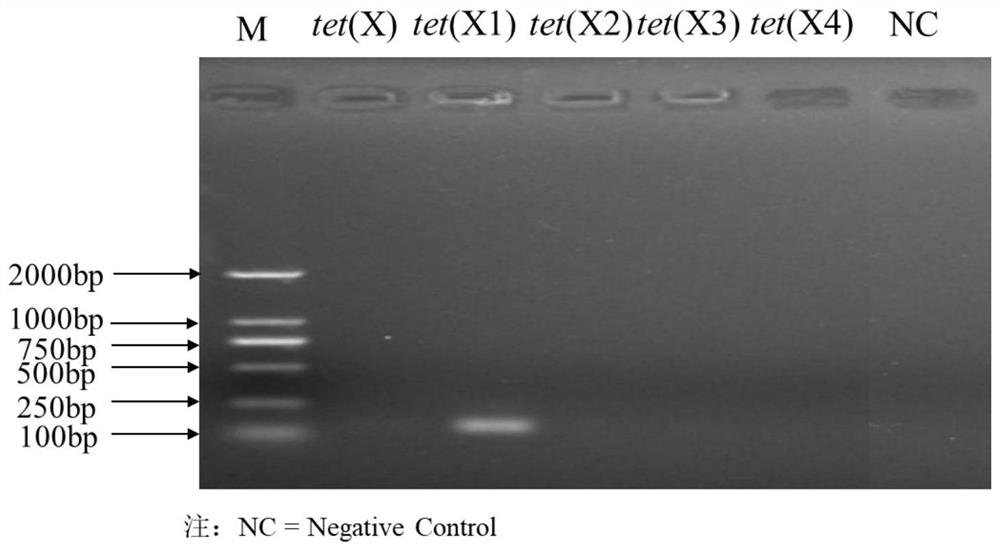
Tigecycline resistance gene family tetX multiplex PCR detection kit
ActiveCN110257509AQuick checkEfficient detectionMicrobiological testing/measurementTigecyclineMicrobiology
The invention provides a tigecycline resistance gene family tetX multiplex PCR detection kit. The tigecycline resistance gene family tetX multiplex PCR detection kit consists of a rapid PCR mixed solution, ultrapure water, a primer probe mixed solution, a standard substance and a negative reference substance, wherein the primer probe mixed solution comprises primer pairs of tetX1, tetX2, tetX3, tetX4, tetX5 and the like. According to the invention, a sensitive and efficient PCR detection method is established by using multiplex PCR test means, and strains containing tetX1, tetX2, tetX3, tetX4 and tetX5 genes can be rapidly and effectively detected. The tigecycline resistance gene family tetX multiplex PCR detection kit has the advantages of low cost, high efficiency, convenience in operation, high accuracy, less energy consumption, less environmental pollution and the like.
Owner:ZHEJIANG UNIV
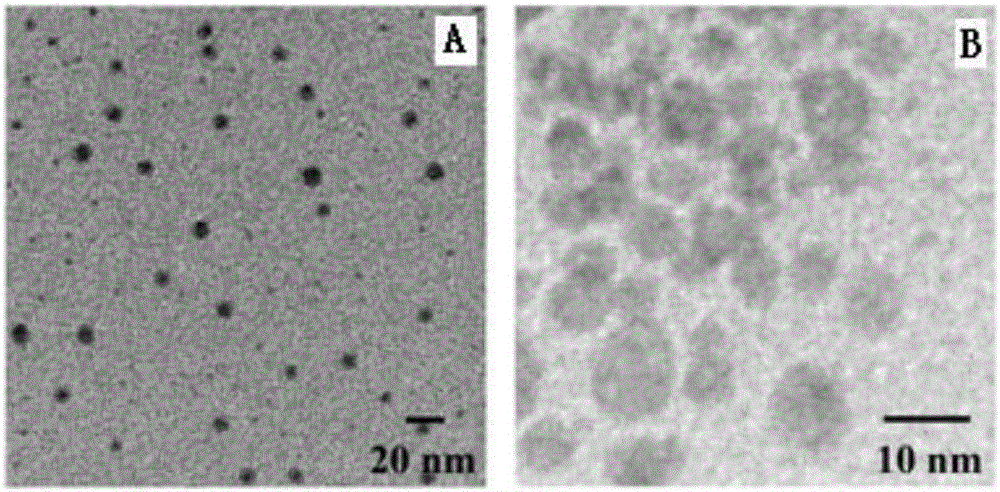
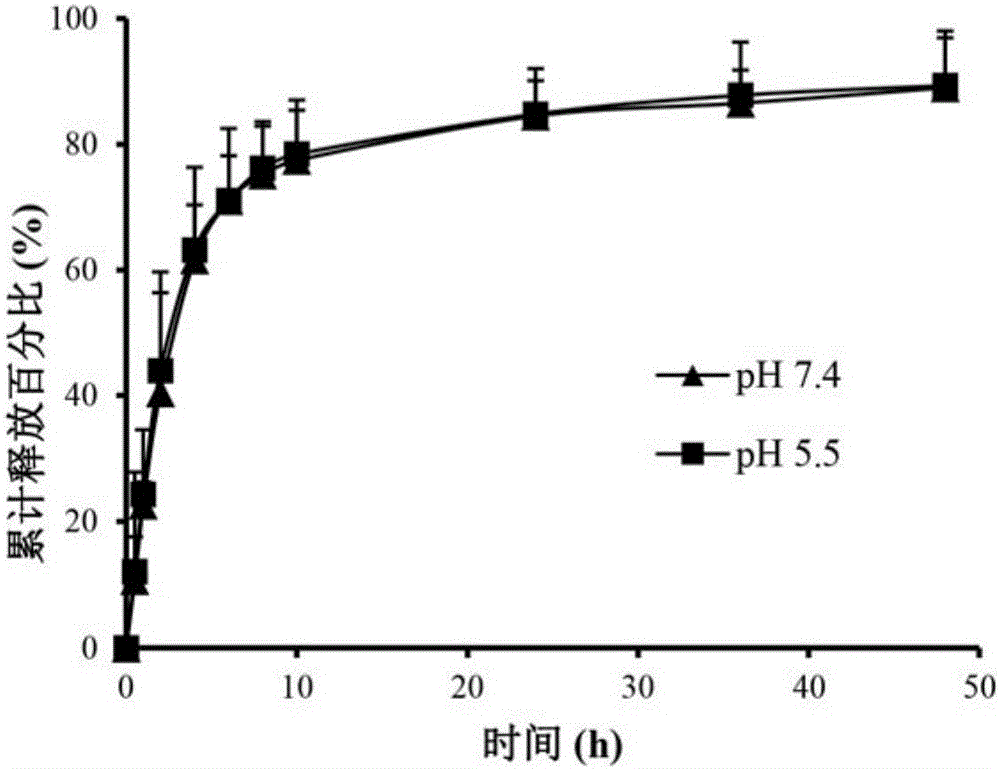
Polyethylene glycol vitamin E succinate modified tigecycline loading silver nanoparticles as well as preparation and application
ActiveCN106727431AStable, safe and synergistic antibacterial effectReduce concentrationAntibacterial agentsInorganic active ingredientsEscherichia coliMinimum inhibitory concentration
The invention provides polyethylene glycol vitamin E succinate modified tigecycline loading silver nanoparticles. First, under the stabilizing effect of polyethylene glycol vitamin E succinate, hollow silver nanoparticles are formed through kirkendall effect, and polyethylene glycol vitamin E succinate modified silver nanoparticles are synthesized, thus the stability of the hollow silver nanoparticles is improved, and toxicity caused by oxidation of the silver nanoparticles is reduced. An antibacterial drug tigecycline is effectively loaded by the hollow structure of polyethylene glycol vitamin E succinate modified tigecycline loading silver nanoparticles. With relatively small particle size, the nanoparticles can penetrate through the cell wall of bacteria, destroy cell membrane and enter cytoplasm; and through the synergistic antibacterial effect of silver nanoparticles and tigecycline, the minimal inhibitory concentration and minimal bactericidal concentration of a drug are remarkably reduced, and the drug resistance of escherichia coli against tigecycline is overcome. The silver nanoparticles can serve as a novel antibacterial nano material for preparing antibacterial drug-resistant drugs and is applied to the field of life science and pharmacy.
Owner:ZHEJIANG UNIV
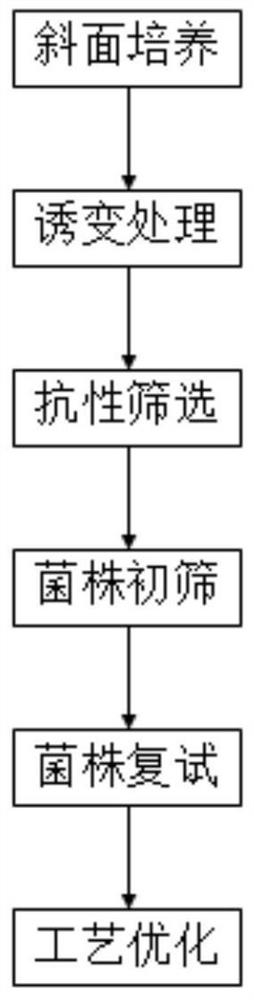
Method for increasing yield of demethyltetracycline
PendingCN111763667AThe synthesis steps are simpleReduce manufacturing costBacteriaMutant preparationBiotechnologyMicroorganism
The invention belongs to the technical field of microbial fermentation, and particularly relates to a method for increasing the yield of demethyltetracycline. The method comprises the following stepsof (1) carrying out slant culture; (2) carrying out mutagenesis treatment; (3) carrying out resistance screening; (4) carrying out strain primary screening; (5) carrying out strain retesting; and (6)carrying out fermentation process optimization on strains obtained by screening in the step (5) to further improve the fermentation level of the demethyltetracycline. Through strain breeding and process optimization, the demethyltetracycline can be produced by using demeclocycline production strains, and the industrial production level is achieved; and the method has important application value for simplifying the synthesis steps of minocycline, tigecycline and other high-added-value products and reducing the production cost.
Owner:HEBEI SHENGXUE DACHENG PHARMA
Bactericide for gray mold of actinidia chinensis
The invention provides a bactericide for gray mold of actinidia chinensis. The bactericide is prepared from the following components by weight: 400-600g of deionized water, 4-6g of emulsifier, 15-25g of chloroisobromine cyanuric acid, 2-4g of cyrtomium rhizome extract, 2-4g of perilla extract, 2-4g of cypress bark extract, 2-4g of derris trifoliata extract, 4-6g of matrine, 4-6g of amoxicillin, 3-5g of clavulanic acid, 2-4g of tigecycline, 4-6g of alpha-cypermethrin, 3-5g of compound sodium nitrophenolate, 2-4g of benzoic acid, 2-4g of copper sulfate, 2-4g of tobramycin, 2-4g of anthocyanin from purple sweet potatoes and 2-4g of ethylparaben. The bactericide is prepared by the steps of heating the deionized water to 50-65 DEG C, putting the rest components into the deionized water, conducting continuous stirring for about 10 minutes, and conducting cooling to normal temperature to obtain the bactericide. By adopting the bactericide, gray mold of actinidia chinensis can be effectively prevented and controlled, and germs can be killed. The bactericide cannot be remained in a plant body under a normal application technical condition, and has small influence on the natural environment and other organisms.
Owner:GUANGDE YUANYE FRUIT GROWING FAMILY FARM
Tigecycline and preparation method thereof
InactiveCN101386582AAdvantage Water SolubilityPredominant antibacterial activityOrganic compound preparationCarboxylic acid amides preparationSolubilityTigecycline
The present invention provides tigecycline and a method for preparing tigecycline salt. The tigecycline salt is obtained through the reaction of tigecycline and a corresponding acid. The tigecycline salt is apparently better in water-solubility and bacteriostasis activity, compared with the tigecycline in a dissociative state, and thus the method has a wide application prospect.
Owner:SHANGHAI LAIYI BIOMEDICAL RES & DEV CENT +1
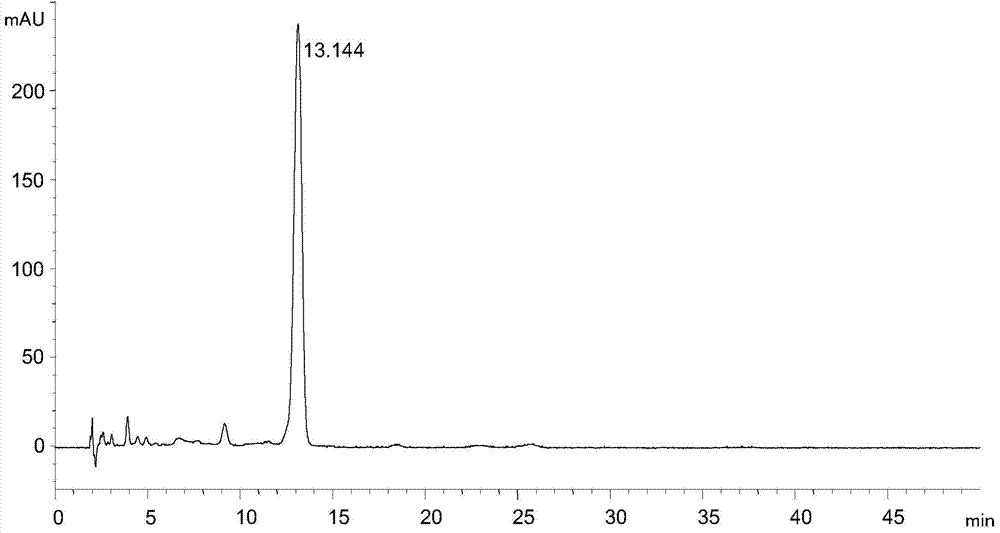
Tigecycline intermediate analysis detection method
ActiveCN104515820AEfficient separationEasy to operateComponent separationTigecyclineColumn temperature
The invention discloses a tigecycline intermediate analysis detection method used for tigecycline intermediate quality control, a chromatographic column with octadecylsilane chemically bonded silica as a packing is used for high performance liquid chromatography analysis detection at the column temperature of 25-35 DEG C in the flow rate of 0.6-1.2mL / min with the detection wavelength of 245nm, wherein the pH value of the mobile phase is adjusted to 6.80-7.20 with 10% ammonia. The tigecycline intermediate analysis detection method selects an ordinary chromatographic column and a liquid chromatographic system, has the advantages of low price, high practicability and simple operation, can effectively separate a tigecycline intermediate from impurities.
Owner:SHANDONG NEWTIME PHARMA
Process for the preparation of tigecycline in the amorphous form
InactiveUS20090275766A1Organic compound preparationCarboxylic acid amide separation/purificationTigecyclineFreeze-drying
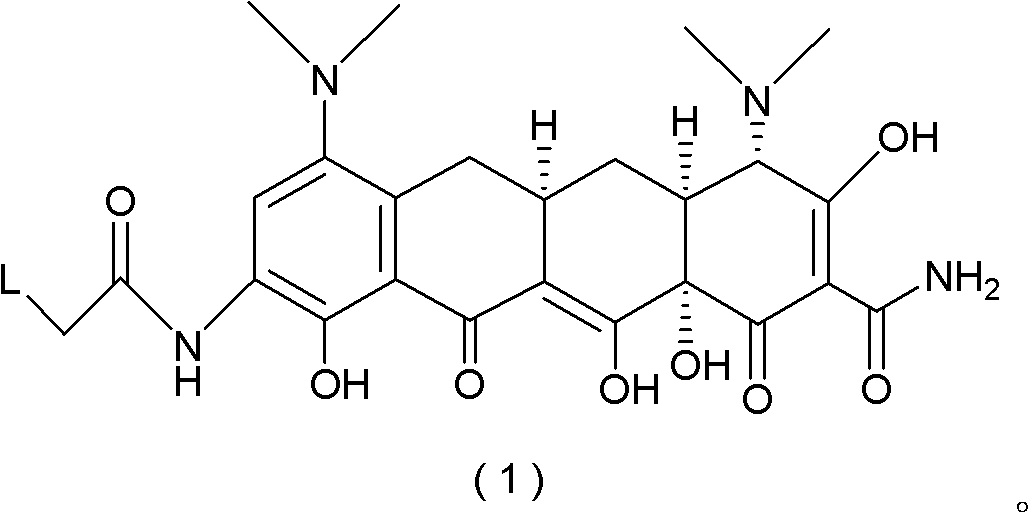
The invention relates to processes for the preparation of tigecycline (I):a wide spectrum antibiotic belonging to the tetracycline family, in stable amorphous form, by freeze-drying, antisolvent precipitation and nebulization.
Owner:ANTIBIOTICOS SPA
Tigecycline formulations
InactiveUS20100035845A1Extended shelf lifeLong-term stabilityAntibacterial agentsBiocideTigecyclineD-Glucose
The invention is directed to a frozen pharmaceutical formulation suitable for administration to a subject parenterally, comprising a therapeutically effective amount of tigecycline and an agent selected from the group consisting of lactose, dextrose, glucose, mannose, sucrose, ribose, xylose and a combination thereof, wherein the formulation in a pre-frozen state at about 22° C. or in an unfrozen state at about 22° C. has a pH in the range of from 4.0 to 5.5. Preferably, the formulation is suitable for storage at or below about −20° C. over a period of at least about 2 months, preferably 6 months, more preferably 26 months. Alternatively, the formulation is suitable for storage at about 22° C. over a period of about 24 hours.
Owner:WYETH LLC
Preparation method of tigecycline
ActiveCN103044280ASimple preparation processSimple and refined processOrganic compound preparationCarboxylic acid amides preparationPotassium nitrateTigecycline
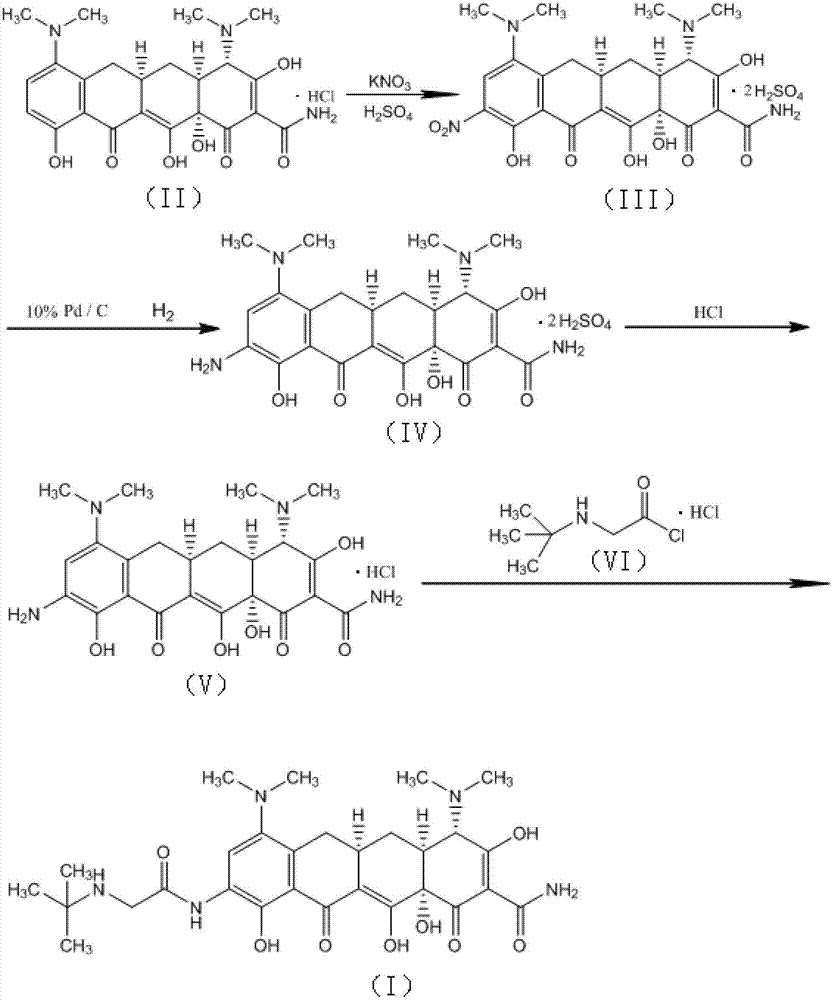
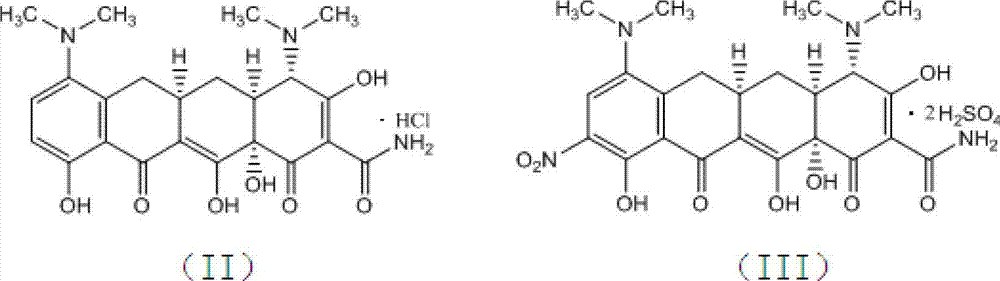
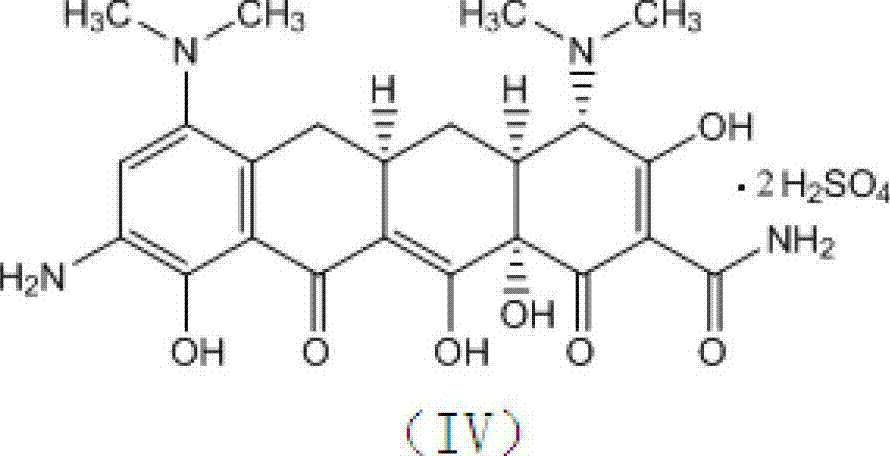
The invention relates to the technical field of medicine synthesis, in particular to a preparation method of tigecycline. The preparation method of the tigecycline comprises the following steps: adding 1,900 mL of concentrated sulfuric acid into a reactor, stirring, cooling to -10 to 15 DEG C, adding 100 g of a compound (II) in batches, and preserving heat and stirring for 20 minutes; controlling the temperature at -10 to 10 DEG C, adding 285-295 g of potassium nitrate in batches, keeping the temperature at 0-5 DEG C, and further stirring for 3-4 h; and slowly dripping a reaction solution into the cooled 4,950-5,050 mL of diethyl ether, controlling the temperature at 0-15 DEG C, further stirring for 10-15 minutes after dripping, separating out light yellow solid, filtering, eluting a filter cake with cold ethanol twice, draining, and drying to obtain a compound (III). The preparation method of the tigecycline is simple and quick in preparation process, relatively high in yield, relatively simple and quick in refining process and relatively high in product purity, and is applicable to industrial production; and by the preparation method of the tigecycline, the reaction time can be effectively shortened.
Owner:ANHUI YOUCARE KAIYUE PHARMA
Method for preparing tigecycline intermediate and salt thereof
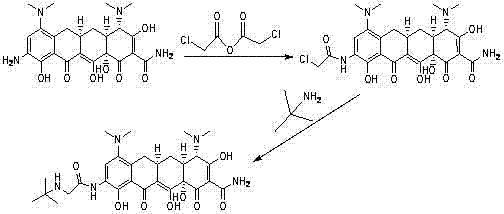
InactiveCN101955443AAvoid it happening againRaw materials are cheap and easy to getOrganic compound preparationCarboxylic acid amides preparationTigecyclineOrtho position
The invention discloses a method for preparing a tigecycline intermediate shown as a formula (1) and a salt thereof, which comprises the preparation route shown in the specifications. The preparation route has the advantages of cheap and readily available raw materials, avoidance of an ortho-position and para-position mixture during nitration, high yield in each step of reaction, no need of a minocycline intermediate, simple and convenient operation and simple equipment requirement, and is a synthesis process for industrially producing tigecycline.
Owner:ZHEJIANG UNIV
Compositions and methods for immunization against drug resistant acinetobacter baumannii
InactiveUS20120301474A1Antibacterial agentsImmunoglobulins against bacteriaPassive ImmunizationsPan drug resistant
The present invention provides vaccine compositions comprising OmpA, or antigenic fragments thereof, and related methods of active immunization against A. baumannii infection. The invention also provides antibodies and antigen-binding parts thereof that specifically bind to OmpA, and related methods of passive immunization against A. baumannii infection. The compositions and methods of the invention are useful for preventing or treating A. baumannii infections, including those caused by strains resistant to carbapenems and all other antibiotics except colistin or tigecycline, also referred to as extreme drug resistant (XDR) A. baumannii infections, and those resistant to every FDA approved antibiotic, also referred to as pan-drug resistant (PDR) A. baumannii infections.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
Methods for detecting impurities in tigecycline
The invention discloses a method for detecting N-tertbutyl glycyl acyl hydrochloride in tigecycline. The invention further discloses a method for detecting N-tertbutyl glycine hydrochloride in tigecycline. The methods are used for detecting the N-tertbutyl glycyl acyl hydrochloride and the N-tertbutyl glycine hydrochloride in tigecycline, are accurate and reliable in detection result, strong in specificity and short in detection time; and moreover a good separation degree of the N-tertbutyl glycyl acyl hydrochloride and the N-tertbutyl glycine hydrochloride is achieved, so that the possibility in comprehensively detecting the impurities in a tigecycline product, controlling the product quality and ensuring the medicine security is provided.
Owner:CHENGDU BAIYU PHARMA CO LTD
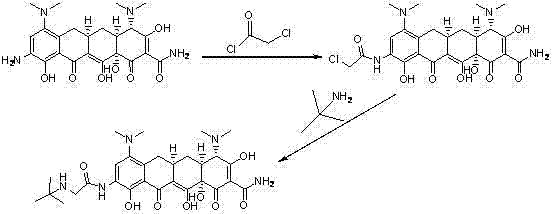
Method for preparing tigecycline intermediate
ActiveCN106831469ASuitable for industrial mass productionEasy to operateOrganic compound preparationCarboxylic acid amides preparationTigecyclineEpimer
The invention relates to a method for preparing a tigecycline intermediate. The tigecycline intermediate can be obtained at a high yield and high purity. The method has the advantages of convenience in operation, safety and environment protection. The obtained product has the characteristics of high purity and low epimer contents and is favorable for industrial amplification.
Owner:JIANGSU HANSOH PHARMA CO LTD
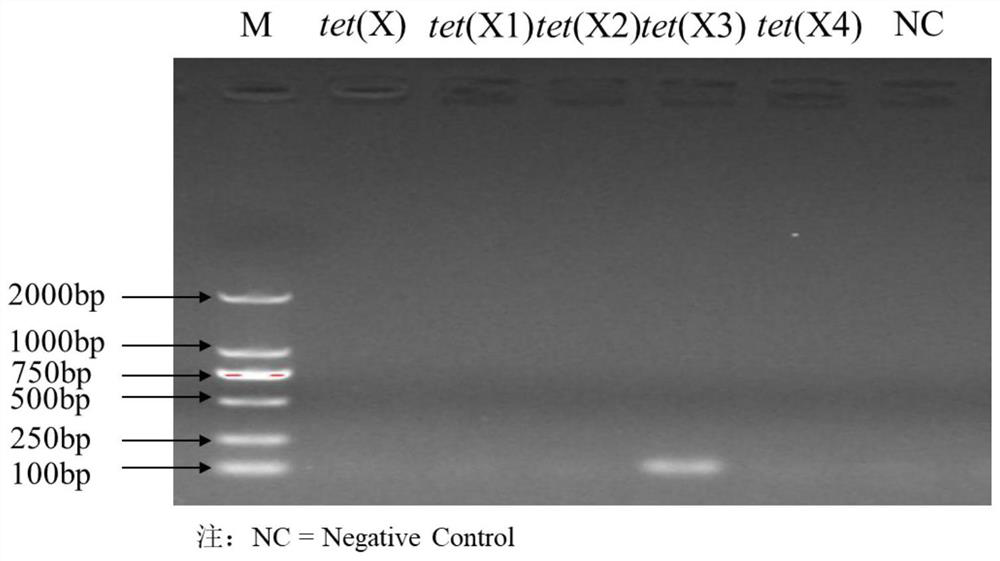
Fluorescent quantitative PCR primer composition for detecting tigecycline drug-resistant gene tet(X) and variants thereof and application of fluorescent quantitative PCR primer composition
PendingCN112760390AMicrobiological testing/measurementDNA/RNA fragmentationResistant genesTigecycline
The invention discloses a fluorescent quantitative PCR primer composition for detecting a tigecycline drug-resistant gene tet(X) and variants thereof and an application of the fluorescent quantitative PCR primer composition. A corresponding fluorescent quantitative PCR method is established for five variants tet(X), tet(X1), tet(X2), tet(X3) and tet(X4) of the tigecycline drug-resistant gene tet(X), the detection method is suitable for culturable bacteria and non-culturable bacteria, and the PCR reaction conditions are the same, so that the five variants of the tigecycline drug-resistant gene tet(X) in a sample can be detected at the same time under the same PCR condition, and the fluorescent quantitative PCR primer composition has important significance on monitoring the drug resistance of antibiotic tigecycline in the environment.
Owner:CHINA AGRI UNIV
Manufacturing process for tigecycline
Disclosed herein is a manufacturing process for the preparation of tigecycline suitable for intravenous infusion.
Owner:WYETH LLC
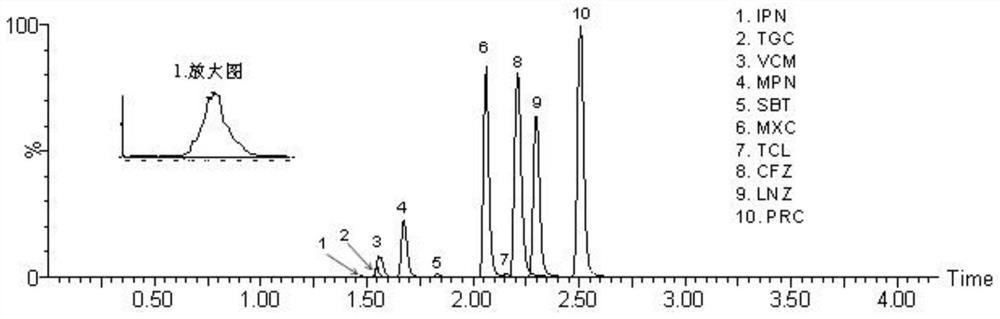
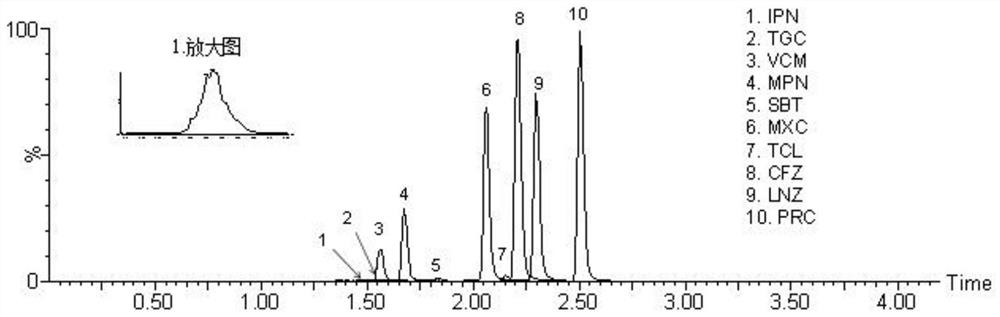
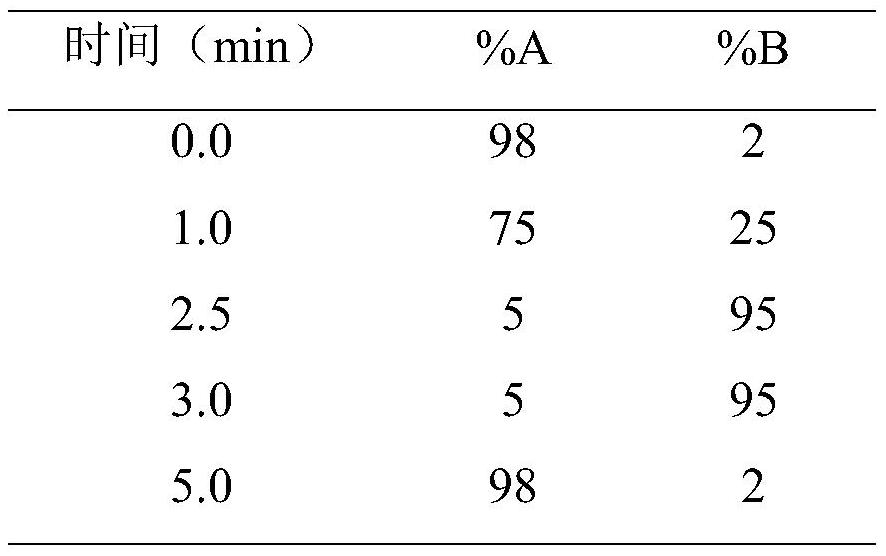
Method for detecting antibacterial agent in serum by ultra-high performance liquid chromatography-tandem mass spectrometry technology
The invention discloses a method for detecting an antibacterial agent in serum by an ultra-high performance liquid chromatography-tandem mass spectrometry technology. The antibacterial agent containssulbactam (SBT), imipenem (IPN), linezolid (LNZ), melopenem (MPN), moxifloxacin (MXC), piperacillin (PRC), tigecycline (TGC), cefoperazone (CFZ), vancomycin (VCM) and teicoplanin (TCL). The method comprises the steps: detecting the content of the antibacterial drug in the pretreated serum by adopting an ultra-high performance liquid chromatography tandem mass spectrometry method, quantifying by utilizing a mass spectrometry isotope internal standard method, establishing a calibration curve by taking the concentration ratio of a standard substance to an internal standard substance as an X axisand the peak area ratio of the standard substance to the internal standard substance as a Y axis, and calculating the concentration of a target drug in the serum. According to the method, the pretreatment process is simple, the sensitivity is high, the specificity is high, separation and detection of the antibacterial agent are completed within 5 min, and a reliable detection method is provided for monitoring the treatment concentration of the antibacterial agent clinically.
Owner:南京品生医学检验实验室有限公司
Production method of tigecycline raw medicinal material
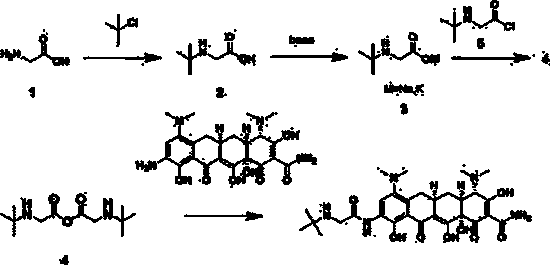
ActiveCN104230744AReduce contentAvoid prone toOrganic compound preparationCarboxylic acid amides preparationGlycineBiochemical engineering
The invention discloses a production method of a tigecycline raw medicinal material and particularly relates to a method which comprises the following steps: by using glycine as a raw material, carrying out a reaction with 2-chloro-2-methylpropane to prepare tertiary butyl glycine; then forming corresponding salt with an inorganic alkali and reacting with tertiary butyl acyl chloride to generate anhydride; and carrying out a condensation reaction on anhydride and 9-amino minocyline to prepare tigecycline. The production method of the tigecycline raw medicinal material disclosed by the invention has the advantages that the raw material is low in price and easily available, and products with high purity and high yield are obtained by adjusting a synthetic route.
Owner:HUNAN ER KANG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com