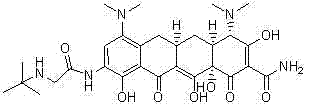
Synthetic method for high-purity tigecycline
A technology of tigecycline and its synthesis method, which is applied in the fields of chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., and can solve problems such as difficulty in purity, high adverse reactions of patients, difficulty in meeting the quality requirements of injection raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation of Tigecycline
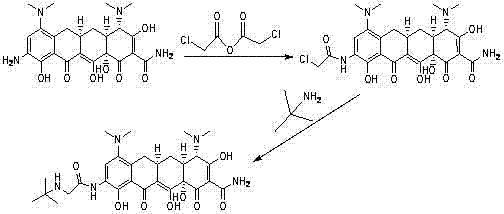
[0031] Add 4.2g (0.025mol) of N-tert-butylglycine to a 500ml three-necked flask, add 126ml of dichloromethane, and add the condensing agent HOAt (4.1g, 0.03mol) and triethylamine (7.6g, 0.075mol) under stirring After 30 minutes, 9-aminominocycline (12.7 g, 0.025 mol) was added, and the reaction was stirred at 35-40° C. for 3 h. HPLC monitors that the content of 9-aminominocycline in the reaction solution is less than 1.0% (area normalized), adjust the pH of the reaction solution to 3.0-4.5 with 1mol / L hydrochloric acid solution, separate the organic layer, adjust the pH to 7.0-7.5 with ammonia water, The aqueous phase was extracted with a mixed solvent of dichloromethane / methanol (250ml*3), and the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 8.2 g of crude tigecycline.
[0032] The obtained crude product was dissolved in 82ml of acetone, at 38-45°C, slowly added 82ml of ...
Embodiment 2
[0036] Example 2 Preparation of Tigecycline
[0037] Add 6.3g (0.038mol) of N-tert-butylglycine into a 500ml three-necked flask, add 150ml of dichloromethane, and add the condensing agent CDI (9.2g, 0.057mol) and N,N-diisopropylethylamine under stirring (14.7g, 0.114mol), after 20 minutes, 9-aminominocycline (19.3g, 0.038mol) was added, and the reaction was stirred at 10°C for 6h. HPLC monitors that the content of 9-aminominocycline in the reaction solution is less than 1.0% (area normalized), adjust the pH of the reaction solution to 3.0-4.5 with 1mol / L hydrochloric acid solution, separate the organic layer, adjust the pH to 7.0-7.5 with ammonia water, The aqueous phase was extracted with a mixed solvent of dichloromethane / methanol (250ml*3), and the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 10.1 g of crude tigecycline.
[0038] The obtained crude product was dissolved in 100ml of acetone, at 38-45°C, slowly add...
Embodiment 3
[0039] Example 3 Preparation of Tigecycline
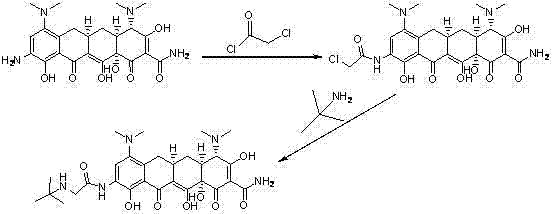
[0040] Add 4.2g (0.025mol) of N-tert-butylglycine to a 500ml three-necked flask, add 63ml of N,N-dimethylformamide, add the condensing agent HATU (5.23g, 0.014mol) and triethylamine ( 2.53g, 0.025mol), after 30 minutes, 9-aminominocycline (6.4g, 0.013mol) was added, and the reaction was stirred at 25°C for 3h. HPLC monitors that the content of 9-aminominocycline in the reaction solution is less than 1.0% (area normalized), adjust the pH of the reaction solution to 3.0-4.5 with 1mol / L hydrochloric acid solution, separate the organic layer, adjust the pH to 7.0-7.5 with ammonia water, Extract the aqueous phase with dichloromethane / methanol mixed solvent (250ml*3), combine the organic phases, dry over anhydrous sodium sulfate, filter, and concentrate to obtain 4.1g of crude tigecycline.
[0041] The obtained crude product was dissolved in 40ml of acetone, at 38-45°C, slowly added 40ml of methanol solution containing 2% lactose, add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com