Method for preparing tigecycline intermediate and salt thereof
A technology for tigecycline and intermediates, which is applied in the field of organic synthesis of drugs, can solve the problems of large impurity content of the 4-position epimer, many impurities, and high cost, and achieve easy-to-obtain raw materials, simple equipment requirements, and easy operation Effect
Inactive Publication Date: 2011-01-26
ZHEJIANG UNIV
View PDF9 Cites 4 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The first method not only has a lower yield (50-60%), but also has more impurities, especially the 4-position epimer impurity content caused by the reaction is relatively large
Although the second method has a yield of 90% in this step of chloroacetylation, the yield of reacting with tert-butylamine is also low (50%), and the route is relatively long simultaneously
This also makes the above-mentioned three synthetic routes of tigecycline subject to the restriction of raw material minocycline, and the cost is higher
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 19
Embodiment 29
Embodiment 39
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
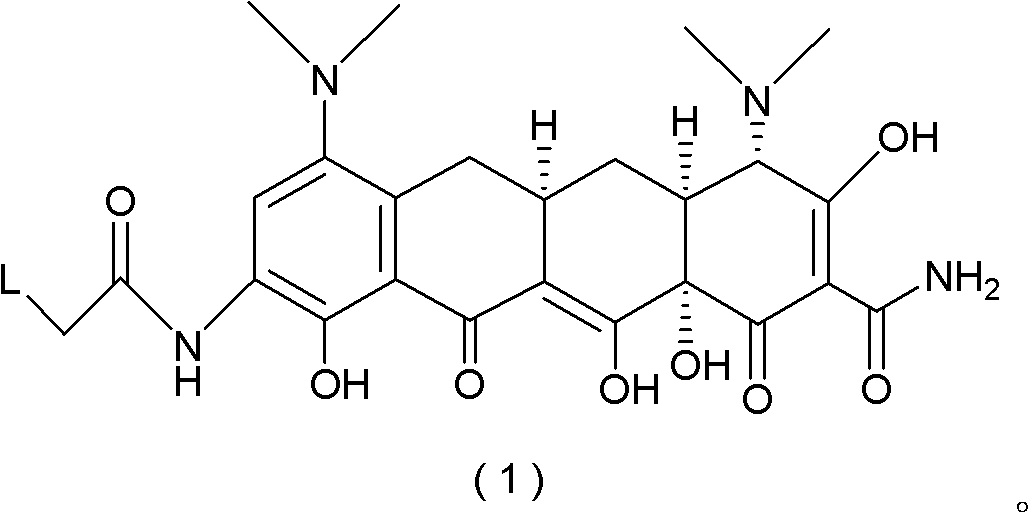
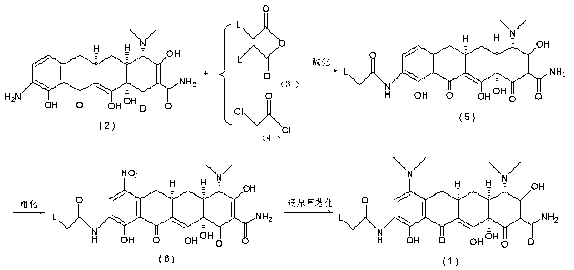
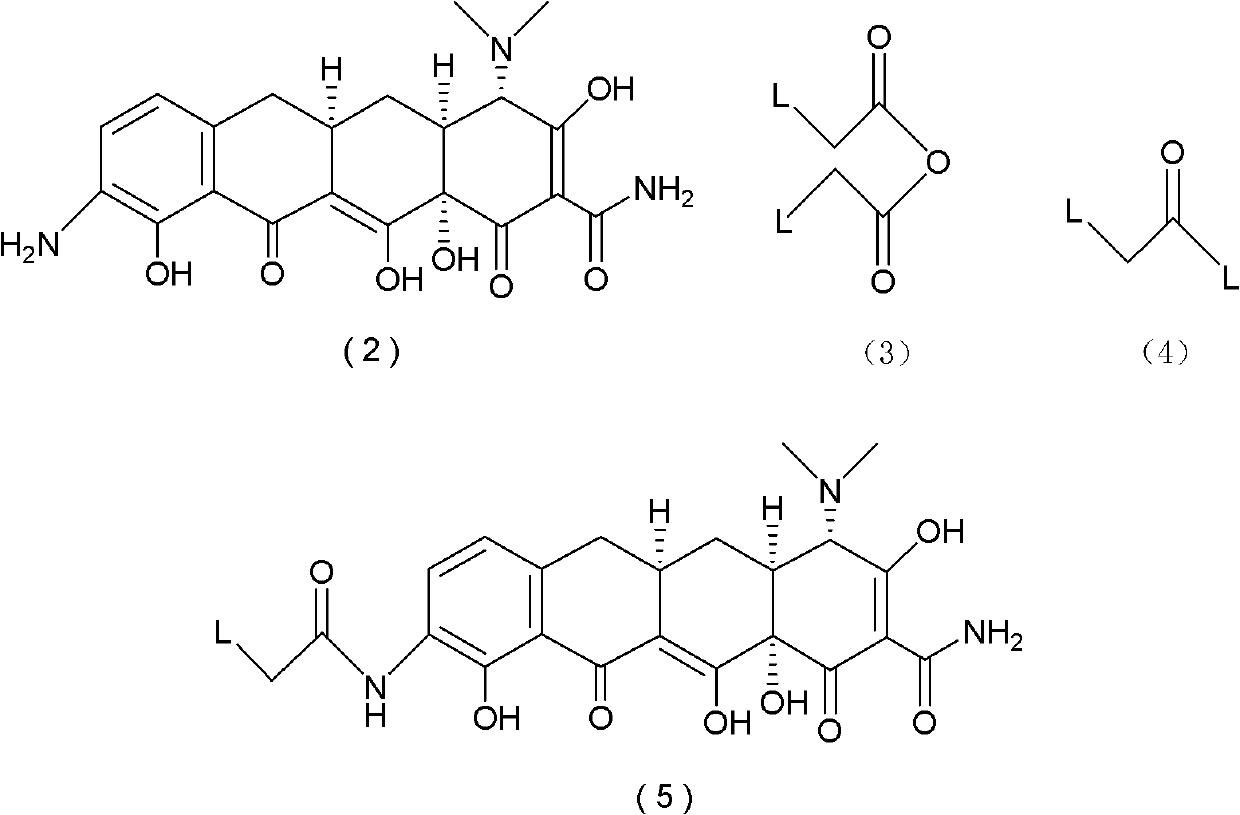
The invention discloses a method for preparing a tigecycline intermediate shown as a formula (1) and a salt thereof, which comprises the preparation route shown in the specifications. The preparation route has the advantages of cheap and readily available raw materials, avoidance of an ortho-position and para-position mixture during nitration, high yield in each step of reaction, no need of a minocycline intermediate, simple and convenient operation and simple equipment requirement, and is a synthesis process for industrially producing tigecycline.
Description
technical field The invention relates to the field of organic synthesis of medicines, in particular to a process for synthesizing tigecycline intermediates and salts thereof. Background technique The chemical name of Tigecycline is [4S-(4α,12aα)]-4,7-bis(dimethylamino)-9-[(tert-butylamino)acetamido]-1,4,4a , 5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-tetracenecarboxamide, its structural formula is as follows: (10) Tigecycline Tigecycline is the first marketed variety of a new class of antibiotics—glycylcycline (glycylcycline), which is mainly used to treat infections caused by Gram-negative and positive pathogenic bacteria, anaerobic bacteria, and methicillin-resistant grapevine aureus. Complicated intra-abdominal infection, skin and skin tissue infection (cSSSI), pneumococcal infection, etc. This product was successfully developed by Wyeth, and was approved for marketing in the United States for the first time in July 2005. Clinical medical resear...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07C237/26C07C231/12
Inventor 杨健余长泉
Owner ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com