Tigecycline without crystal habit and method of preparing the same
A tigecycline, crystal-free technology, used in organic chemistry, separation/purification of carboxylic acid amides, etc., can solve problems such as tigecycline that has not yet seen a crystal-free form, and achieve the effect of obvious bacteriostatic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 , the preparation of tigecycline without crystalline form
[0029] Weigh 0.2 g of crude tigecycline (content about 70%), dissolve it completely with 40 ml of water, and then adjust the pH of the solution to 2.0 with 1 mol / L HCl;
[0030] Add 20ml of HP2MG macroporous adsorption resin (Mitsubishi Chemical) to the solution, stir and adsorb at 100rpm for 1 hour, filter to remove the resin, adjust the pH of the adsorption residue to 8.5 with 1mol / L NaOH; then use dichloromethane and acetonitrile 2:1 to form The mixed solvent was extracted twice, 40ml each time, and the extracts were collected and combined;
[0031] The collected extract was concentrated and dried under vacuum at 20°C to obtain 0.08 g of tigecycline powder.
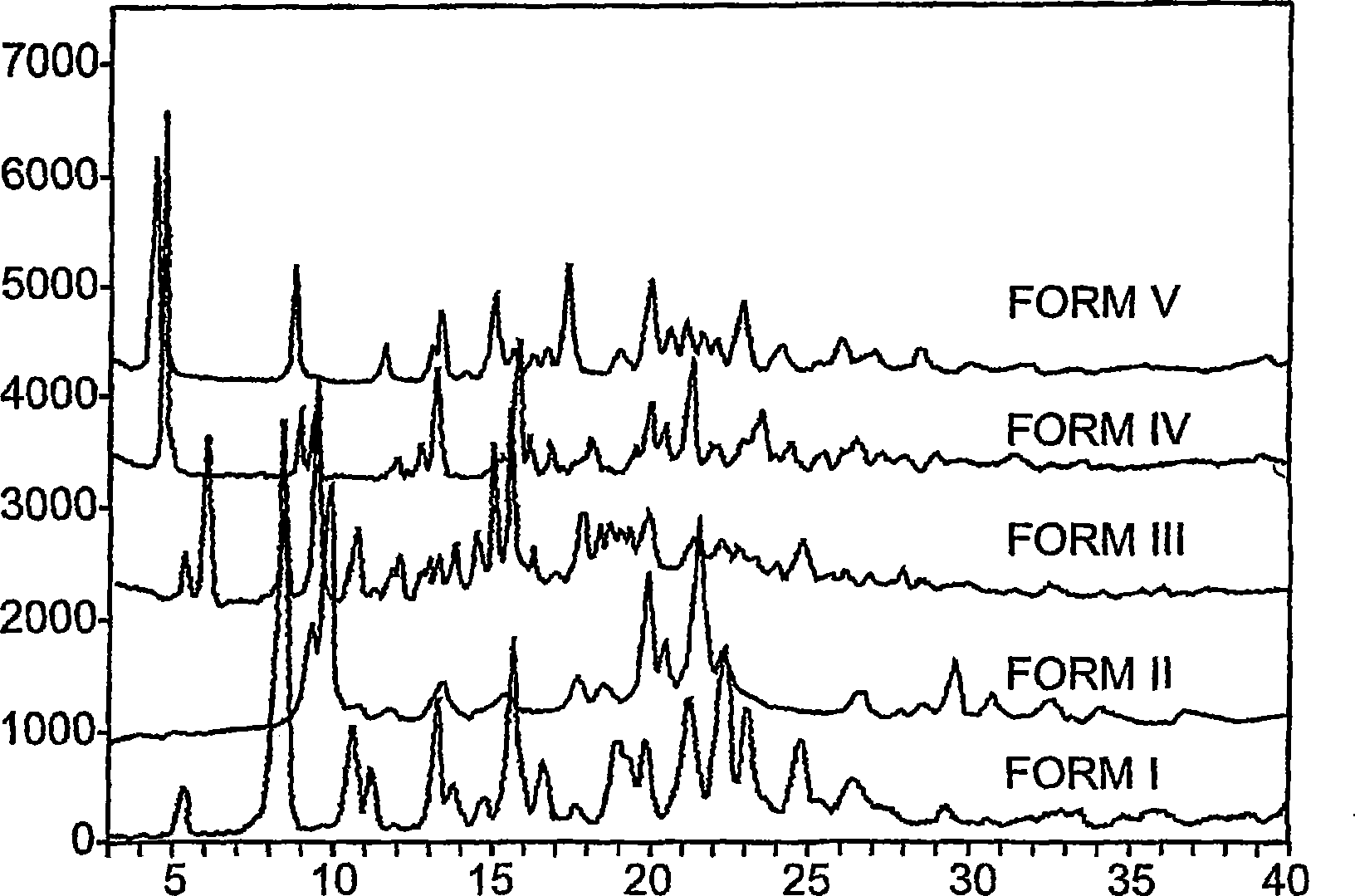
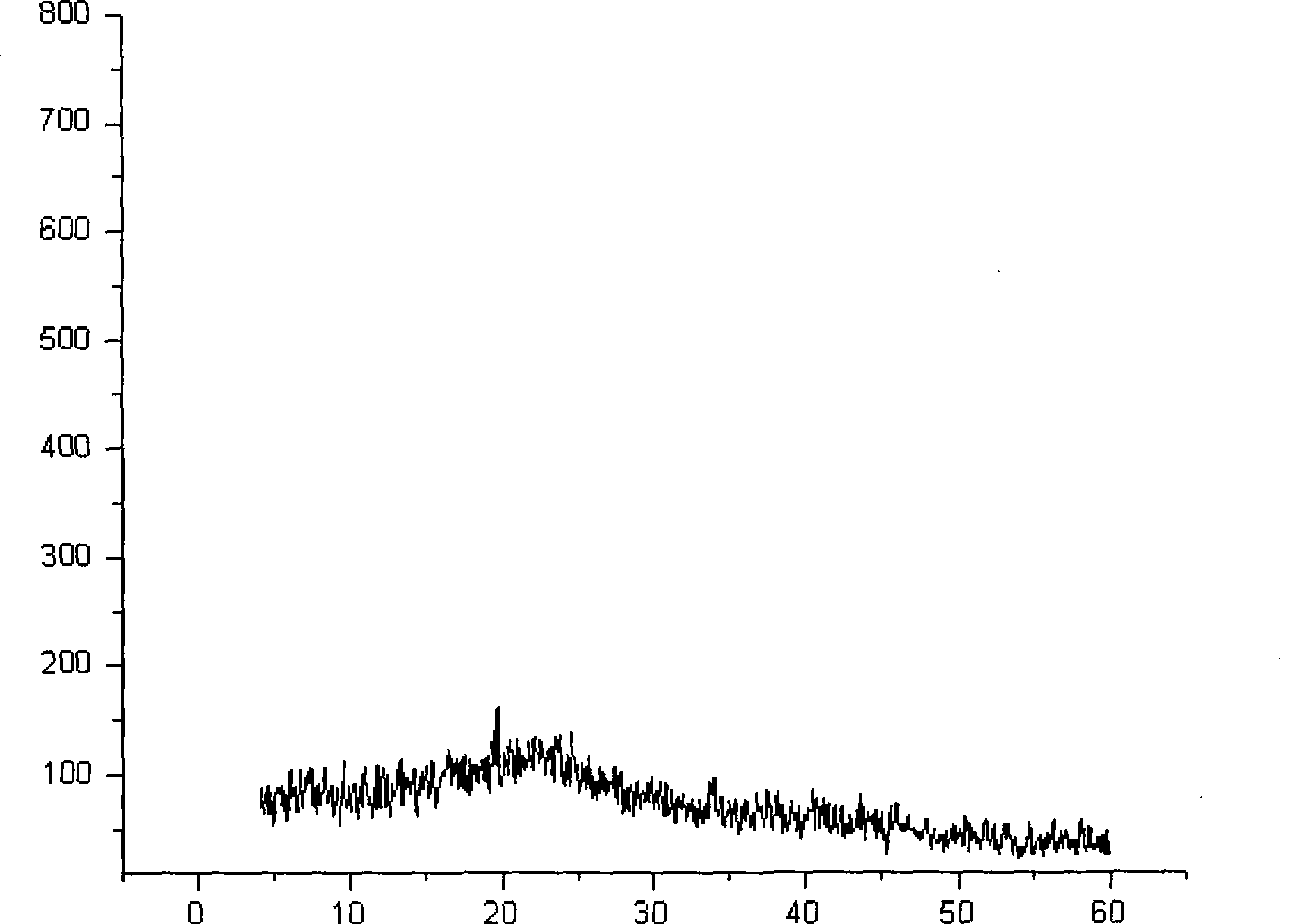
[0032] Use the X-ray powder diffraction method to detect the powder obtained, and the diffraction pattern is as follows figure 2 shown. Depend on figure 2 As can be seen from the results, the diffraction pattern shows a flat curve and ...
Embodiment 2
[0037] Example 2 , the preparation of tigecycline without crystalline form
[0038] Weigh 5.4g of crude tigecycline (content about 70%), dissolve it completely with 800ml of water, and then adjust the pH of the solution to 3.0 with 1mol / L HCl;
[0039] Add 400ml of prepared Amberlite XAD-6 macroporous adsorption resin (Rohm and Haas, USA) to the solution, stir and adsorb at 100rpm for 1 hour, remove the resin by filtration, and adjust the pH of the adsorption raffinate to 8.0 with 1mol / L NaOH; Extract twice with methane, 800ml each time, collect and combine the extracts;
[0040] The collected extract was concentrated and dried under vacuum at 30° C. to obtain 2.3 g of tigecycline powder.
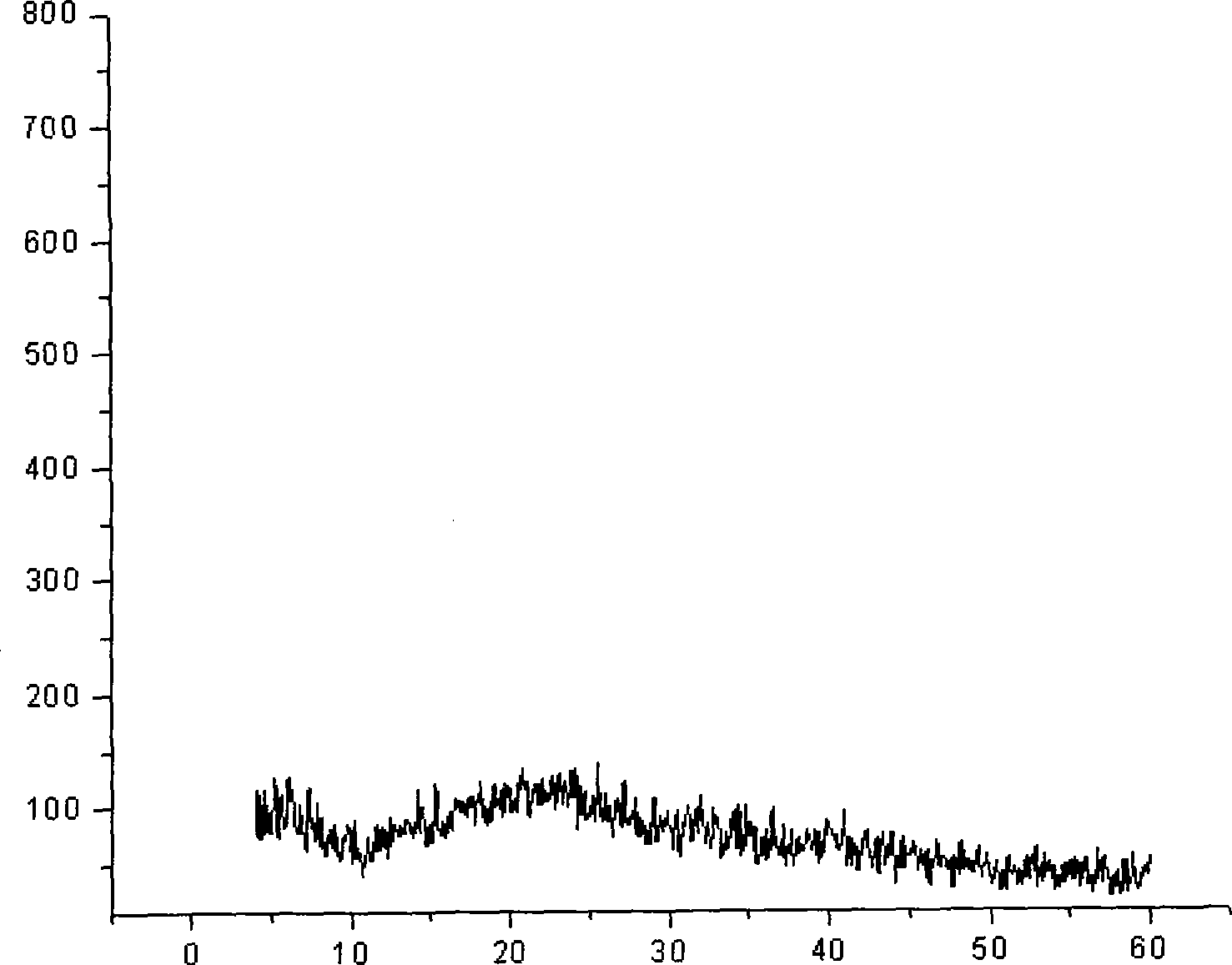
[0041] The obtained powder sample is detected by X-ray powder diffraction method, and the diffraction pattern is as follows image 3 shown. Depend on image 3 As can be seen from the results, the diffraction pattern shows a flat curve and does not show any peak shape, so it can be det...
Embodiment 3
[0043] Example 3 , the preparation of tigecycline without crystalline form
[0044] Weigh 40 g of crude tigecycline (content about 70%), dissolve it completely with 4000 ml of water, and then adjust the pH of the solution to 2.5 with 1 mol / L HCl;
[0045] Add 2000ml of prepared HP2MG macroporous adsorption resin (Mitsubishi Chemical) to the solution, stir and adsorb at 100rpm for 1 hour, filter to remove the resin, and adjust the pH of the adsorption raffinate to 8.3 with 1mol / L NaOH;
[0046] Then extract twice with the mixed solvent that dichloromethane and methanol 3:1 constitute, each 4000ml, collect and combine extract;
[0047] The collected extract was concentrated and dried under vacuum at 15°C to obtain 18.0 g of tigecycline powder.
[0048] Use the X-ray powder diffraction method to detect the powder obtained, and the diffraction pattern is as follows Figure 4 shown. Depend on Figure 4 As can be seen from the results, the diffraction pattern shows a flat curv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com