Preparation method of tigecycline
A technology of adding tigecycline in batches, which is applied in the preparation of organic compounds, carboxylic acid amide preparation, chemical instruments and methods, etc., can solve the problems of harsh reaction conditions, low product purity and low yield, and achieve the goal of preparing The effect of simple process, high product purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
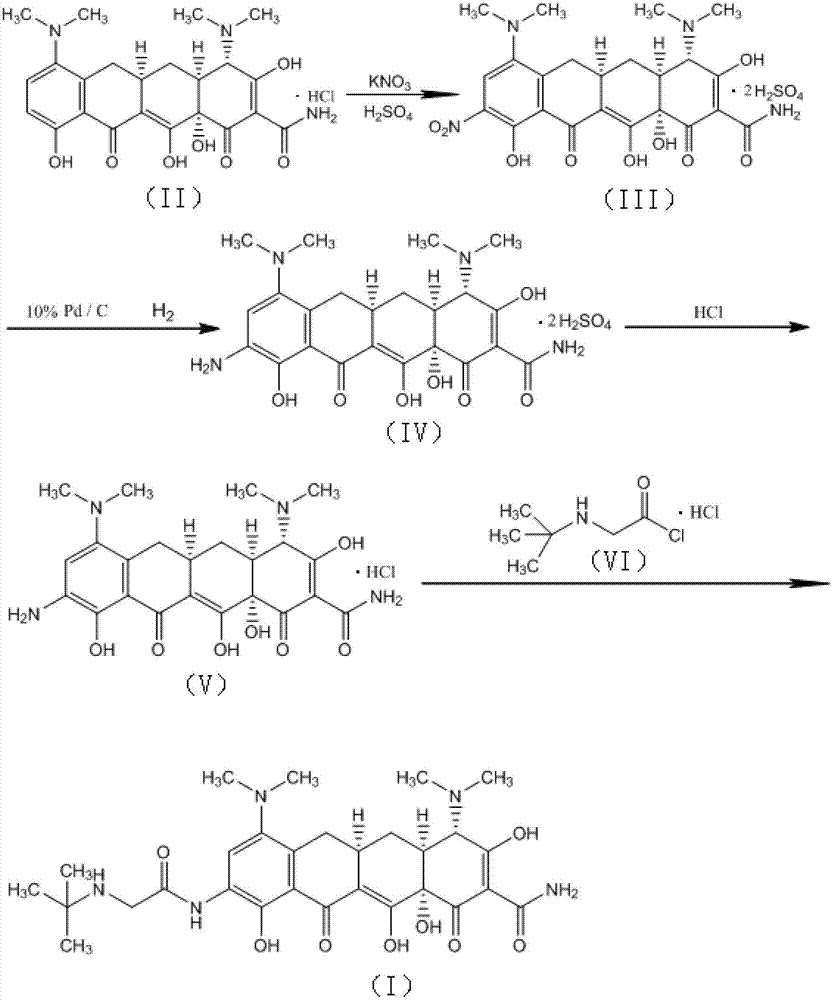
[0027] Below in conjunction with embodiment and attached figure 1 The present invention is further described.
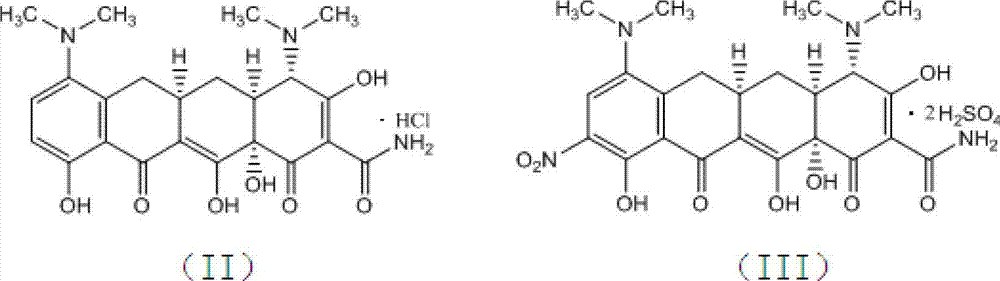
[0028] 1., prepare formula (III) compound by formula (II) compound
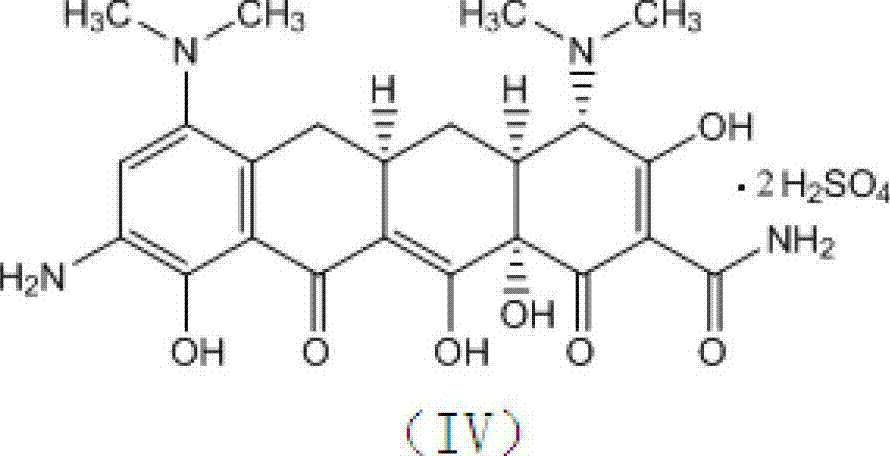
[0029] In a reactor with mechanical stirring, add concentrated sulfuric acid (98%) 1900ml, stir, cool the reaction solution to -10~15°C, add minocycline hydrochloride (compound of formula (II)) 100g in batches, complete, Maintain the temperature and stir for 20 minutes; control the temperature at -10 to 0°C, and then add 290 g of potassium nitrate in batches. After the addition is complete, keep the temperature at 0 to 5°C and continue to stir for 3 hours. Slowly add the reaction solution dropwise into 5000ml of cooled diethyl ether, and control the temperature not to exceed 15°C. After the dropwise addition, continue to stir for 10 minutes, a large amount of light yellow solid precipitates, filter, wash the filter cake twice with cold ethanol, and drain , transferred to a vacuum drying oven at 30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com