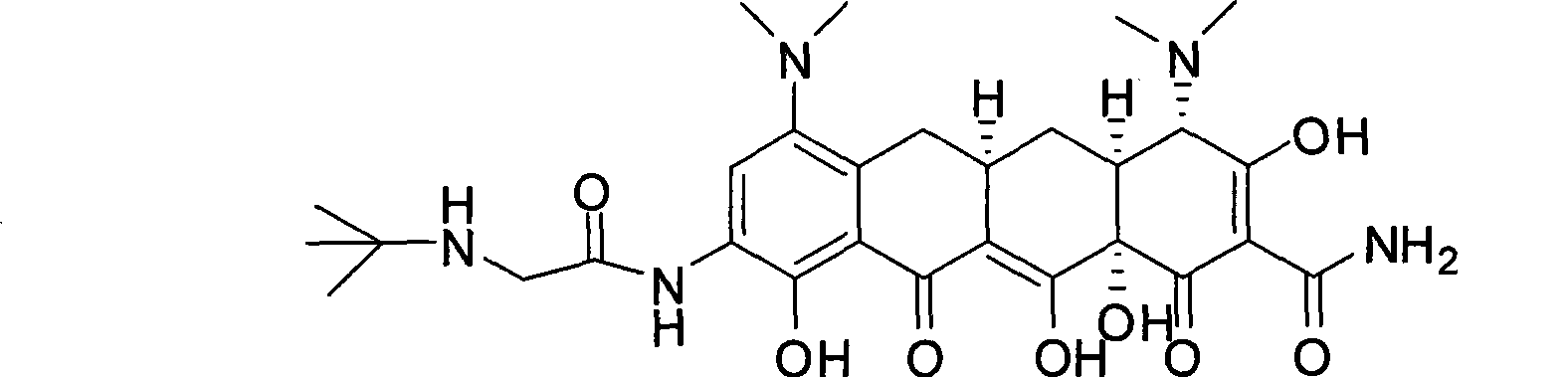
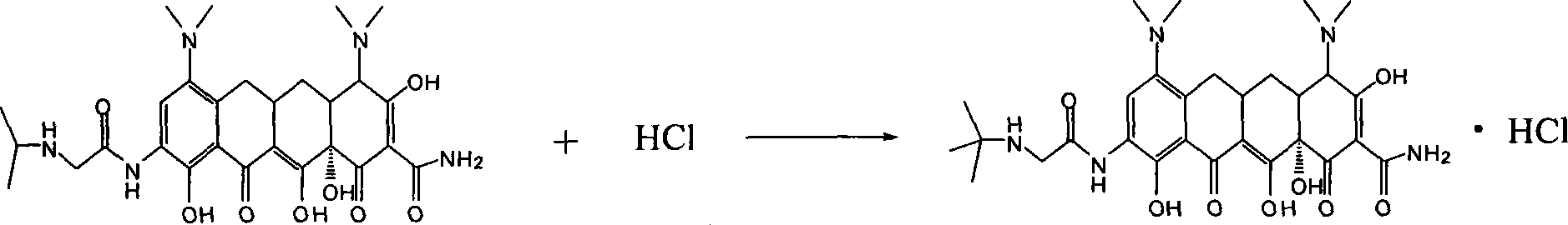Tigecycline and preparation method thereof
A technology of tigecycline and hydrochloride, which is applied to the preparation of carboxylic acid amides, chemical instruments and methods, and the preparation of organic compounds, etc., to achieve the effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1 , Preparation of various acid aqueous solutions
[0015] Preparation of hydrochloric acid aqueous solution: measure 0.8 mL concentrated hydrochloric acid (analytical pure, HCl content 36.0-38.0%), add water to make up to 10 mL.
[0016] Preparation of acetic acid aqueous solution: weigh 0.6g of glacial acetic acid, add water to make up to 10mL.
[0017] Preparation of methanesulfonic acid aqueous solution: Weigh 1.0 g of methanesulfonic acid (analytical pure, content 98%), add water to make up to 10 mL.
[0018] Preparation of aqueous solution of benzenesulfonic acid: weigh 1.6 g of benzenesulfonic acid (analytical pure, content 70%), add water to make up to 10 mL.
[0019] Preparation of lactic acid aqueous solution: Weigh 0.9 g of natural lactic acid (analytical pure, content 99%), add water to make up to 10 mL.
Embodiment 2
[0020] Example 2 , the preparation of tigecycline hydrochloride
[0021] Weigh 0.3 g of tigecycline, dissolve it in 15 mL of water, add 0.5 mL of the hydrochloric acid aqueous solution obtained in Example 1, stir fully to make the two react completely, and the specific reaction formula is as follows:
[0022]
[0023] The reaction solution was frozen in a refrigerator at -20° C. for 5 hours, and then freeze-dried in a lyophilizer overnight to obtain tigecycline hydrochloride powder.
Embodiment 3
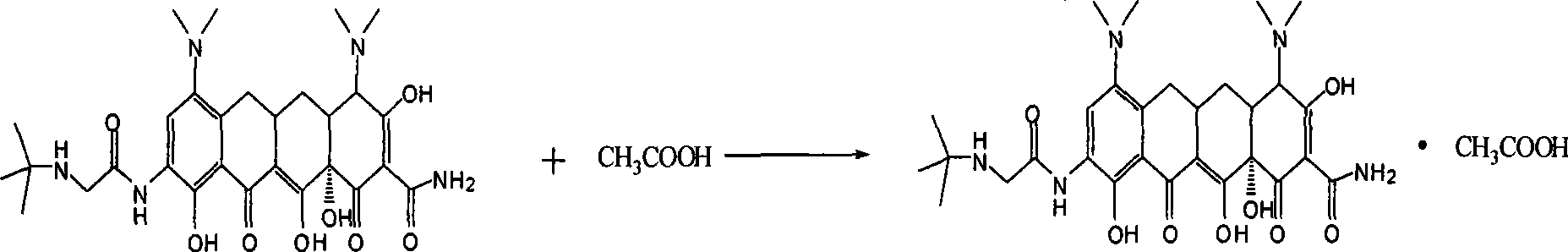
[0024] Example 3 , the preparation of tigecycline acetate
[0025] Weigh 0.3g of tigecycline, dissolve in 15mL of water, add 0.5mL of the aqueous acetic acid obtained in Example 1, fully stir to make the two react completely, and the specific reaction formula is as follows:
[0026]
[0027] The reaction solution was frozen in a refrigerator at -20° C. for 5 hours, and then freeze-dried in a freeze dryer overnight to obtain tigecycline acetate powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com