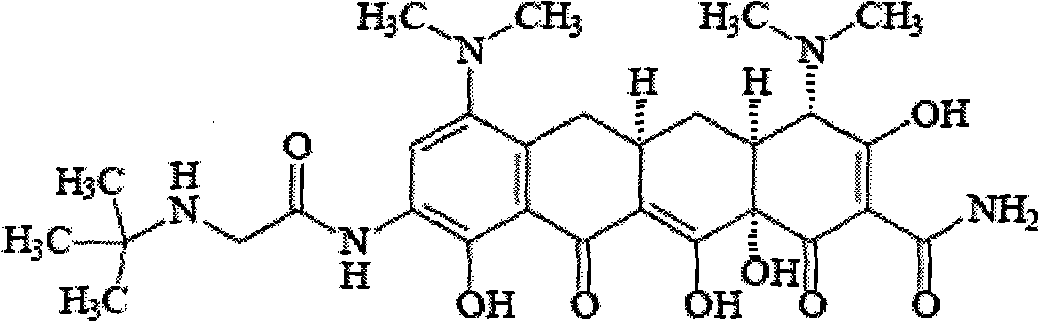
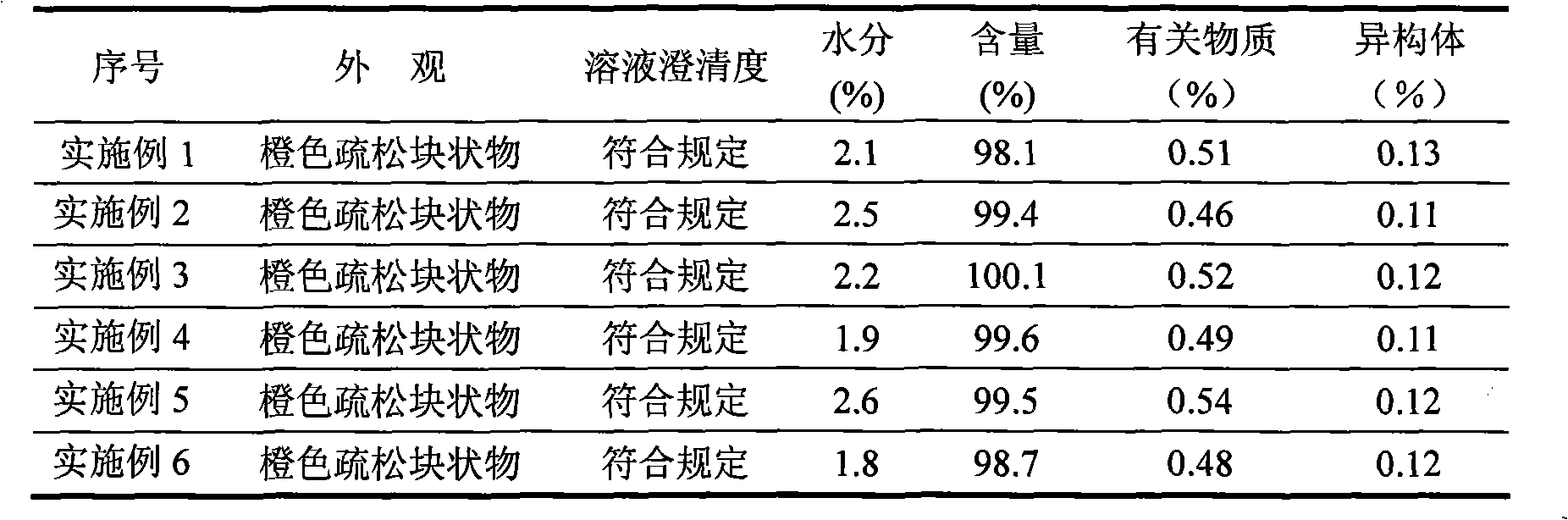
Tigecycline freeze-dried injection
A technology of freeze-dried powder injection and tigecycline, which is applied in the direction of freeze-dried delivery, tetracycline active ingredient, powder delivery, etc. It can solve the problem of strict requirements on preparation and infusion time, inconvenient patient medication safety, tigecycline Poor stability and other problems, to achieve the effect of uniform and stable quality, thorough moisture drying and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] prescription
[0023] Tigecycline 50g
[0024] Dextran 50g
[0025] Anhydrous sodium sulfite 4g
[0026] Sodium citrate 5g
[0027] Add water for injection to 2000ml
[0028]
[0029] A total of 1000 bottles were made
[0030] Weigh the prescribed amount of tigecycline, dextran, sodium citrate and anhydrous sodium sulfite, first put the dextran in a sterile container, add 80% water for injection to dissolve it, and add citrate after the water for injection cools to 4°C Sodium citrate and anhydrous sodium sulfite, dissolve and stir evenly, then add tigecycline, stir to dissolve and mix evenly, adjust the pH to 7.8 with 1mol / L hydrochloric acid or 1mol / L sodium hydroxide solution, then add 0.1 % of activated carbon for needles, stirred and adsorbed for 30 minutes, decarbonized by suction filtration, and after the intermediate inspection was qualified, add water for injection to make up to full volume. The solution is filtered through t...
Embodiment 2
[0032] prescription
[0033] Tigecycline 50g
[0034] Dextran 450g
[0035] Anhydrous sodium sulfite 4g
[0036] Sodium citrate 5g
[0037] Add water for injection to 5000ml
[0038]
[0039] A total of 1000 bottles were made
[0040]Weigh the prescribed amount of tigecycline, dextran, sodium citrate and anhydrous sodium sulfite, first put the dextran in a sterile container, add 80% water for injection to dissolve it, wait for the water for injection to cool to 2°C, then add citrate Sodium citrate and anhydrous sodium sulfite, dissolve and stir evenly, then add tigecycline, stir to dissolve and mix evenly, adjust the pH to 8.0 with 1mol / L hydrochloric acid or 1mol / L sodium hydroxide solution, then add 0.1 % of activated carbon for needles, stirred and adsorbed for 30 minutes, decarbonized by suction filtration, and after the intermediate inspection was qualified, add water for injection to make up to full volume. The solution is filtered through two 0...
Embodiment 3
[0042] prescription
[0043] Tigecycline 50g
[0044] Dextran 200g
[0045] Anhydrous sodium sulfite 10g
[0046] Sodium citrate 10g
[0047] Add water for injection to 3000ml
[0048]
[0049] A total of 1000 bottles were made
[0050] Weigh the prescribed amount of tigecycline, dextran, sodium citrate and anhydrous sodium sulfite, put the dextran in a sterile container, add 80% water for injection to dissolve it, and add citrate after the water for injection is cooled to 6°C Sodium citrate and anhydrous sodium sulfite, dissolve and stir evenly, then add tigecycline, stir to dissolve and mix evenly, adjust the pH to 7.0 with 1mol / L hydrochloric acid or 1mol / L sodium hydroxide solution, then add 0.1 % of activated carbon for needles, stirred and adsorbed for 30 minutes, decarbonized by suction filtration, and after the intermediate inspection was qualified, add water for injection to make up to full volume. The solution is filtered through tw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com