Tigecycline intermediate analysis detection method
A detection method and tigecycline technology, which are applied in the field of high performance liquid chromatography analysis, can solve the problems of expensive chromatographic column, cumbersome method, low practicability, etc., and achieve stable and reliable results, simple operation and high practicability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
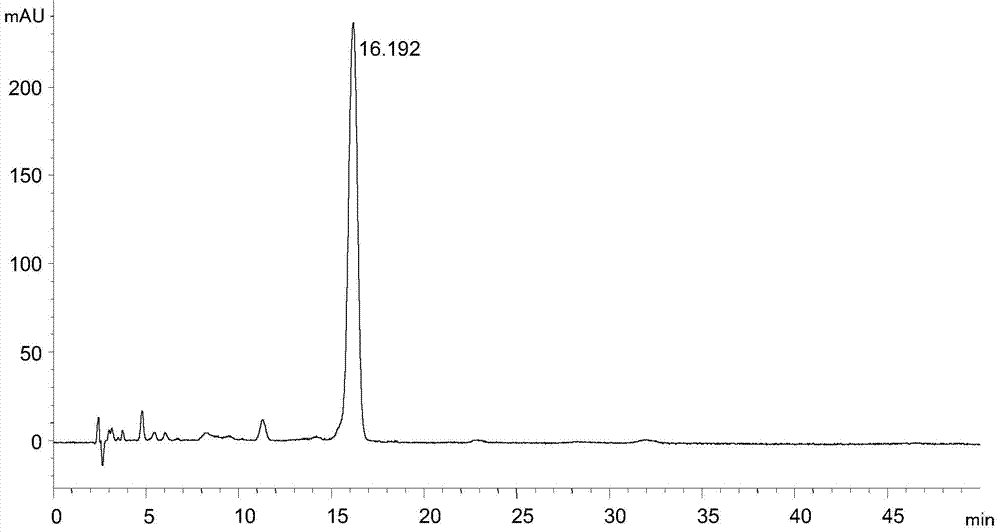
[0019] Instruments and conditions: Agilent1100 liquid chromatography system, chromatographic column: ZORBAX SB-C18 (4.6×250mm, 5μm), detection wavelength 245nm, column temperature 30°C, flow rate 1.0mL / min, mobile phase: 0.05mol / L ammonium acetate solution : N,N-dimethylformamide: trifluoroacetic acid = 60:39.8:0.2, each liter of the mixed solution of the first three contains 0.01mol of ethylenediaminetetraacetic acid, adjust the pH value to 7.02 with 10% ammonia water.
[0020] Experimental procedure: dissolve the tigecycline intermediate with mobile phase and quantitatively dilute to make a solution containing about 0.3 mg of tigecycline intermediate per 1 mL, as the test solution, accurately measure 20 μL of the test solution and inject Liquid chromatograph, carry out high performance liquid chromatographic analysis according to above-mentioned conditions, record chromatogram, the result sees attached figure 1 .
[0021] attached figure 1 It shows that under the chromatog...
Embodiment 2
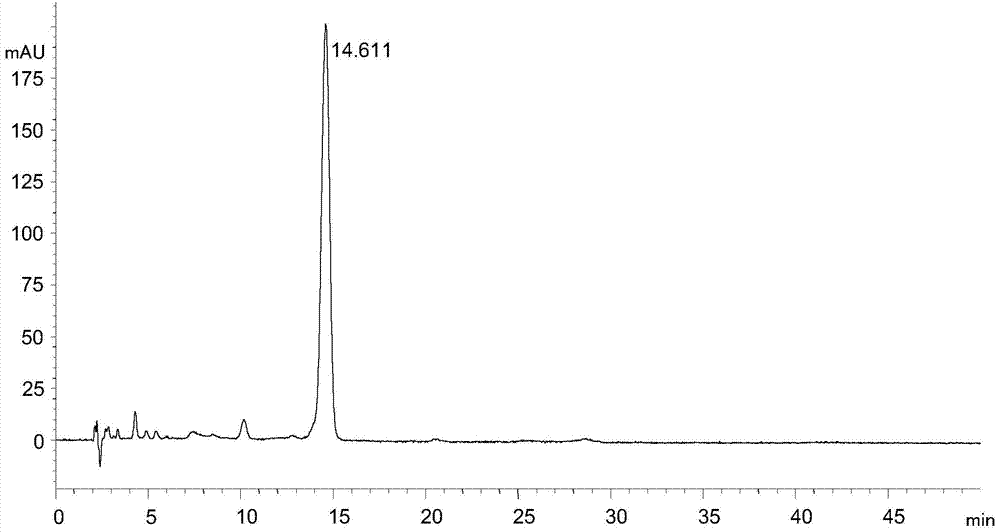
[0023] Instrument and conditions: Agilent1100 liquid chromatography system, chromatographic column: ZORBAX SB-C18 (4.6×250mm, 5μm), detection wavelength 245nm, column temperature 30°C, flow rate 0.6mL / min, mobile phase: 0.05mol / L ammonium acetate solution : N,N-dimethylformamide: trifluoroacetic acid=50:49.8:0.2, each liter of the mixed solution of the first three contains 0.01mol of ethylenediaminetetraacetic acid, adjust the pH value to 7.02 with 10% ammonia water.
[0024] Experimental procedure: dissolve the tigecycline intermediate with mobile phase and quantitatively dilute to make a solution containing about 0.3 mg of tigecycline intermediate per 1 mL, as the test solution, accurately measure 20 μL of the test solution and inject Liquid chromatograph, carry out high performance liquid chromatographic analysis according to above-mentioned conditions, record chromatogram, the result sees attached figure 2 .
[0025] attached figure 2 It shows that under the chromatogr...
Embodiment 3
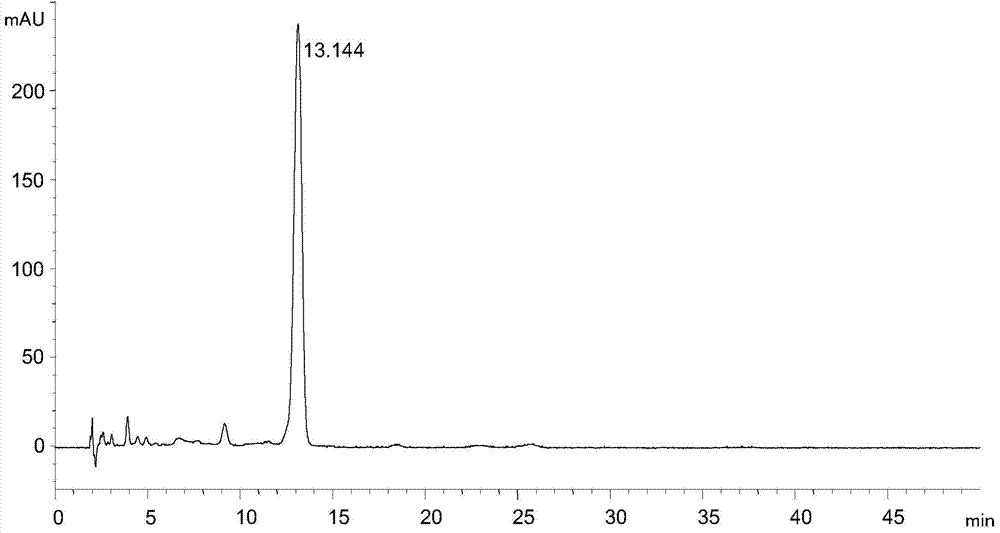
[0027] Instrument and conditions: Agilent1100 liquid chromatography system, chromatographic column: ZORBAX SB-C18 (4.6×250mm, 5μm), detection wavelength 245nm, column temperature 30°C, flow rate 1.2mL / min, mobile phase: 0.05mol / L ammonium acetate solution : N,N-dimethylformamide: trifluoroacetic acid = 70:29.8:0.2, each liter of the mixed solution of the first three contains 0.01mol of ethylenediaminetetraacetic acid, adjust the pH value to 7.02 with 10% ammonia water.
[0028] Experimental procedure: dissolve the tigecycline intermediate with mobile phase and quantitatively dilute to make a solution containing about 0.3 mg of tigecycline intermediate per 1 mL, as the test solution, accurately measure 20 μL of the test solution and inject Liquid chromatograph, carry out high performance liquid chromatographic analysis according to above-mentioned conditions, record chromatogram, the result sees attached image 3 .
[0029] attached image 3 It shows that under the chromatogr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com