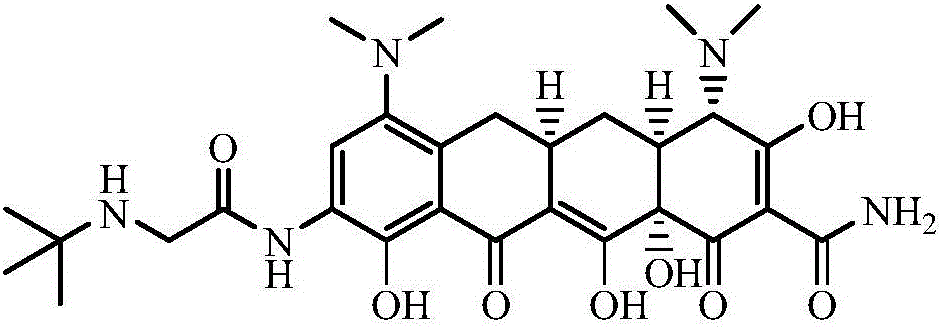
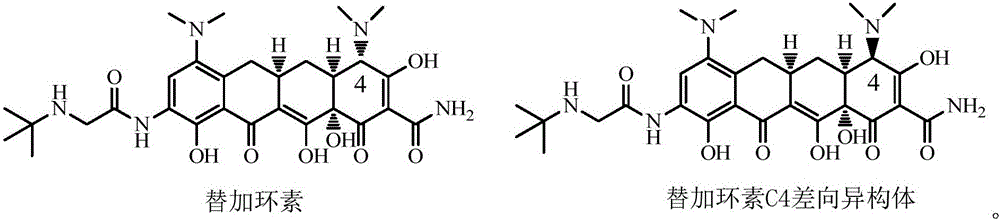
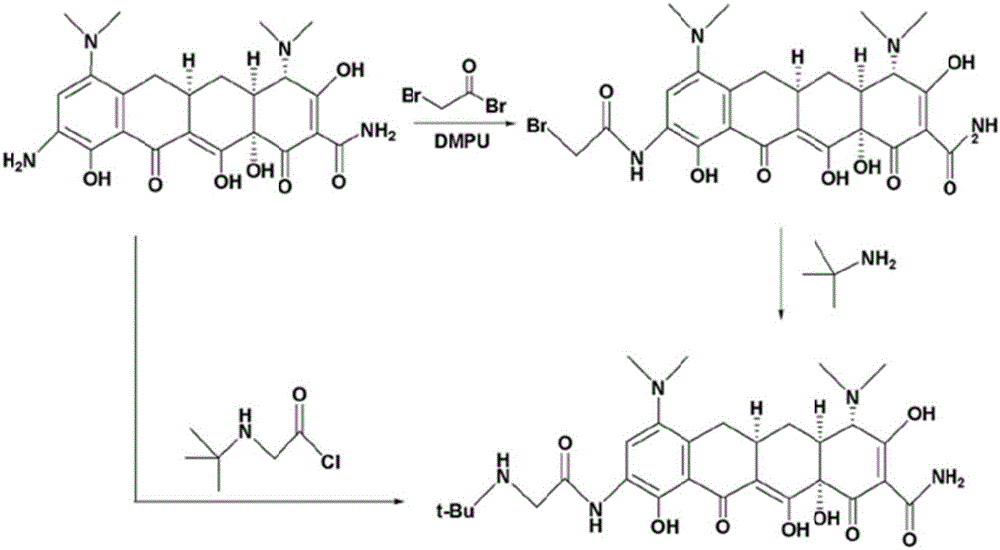
Method for preparing tigecycline intermediate
A Chinese compound technology, applied in the preparation of tigecycline intermediates and 9-nitrominocycline, can solve the problems of low purity, achieve simple operation, reduce the requirements of reaction equipment, and avoid corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add concentrated sulfuric acid (180ml) into the reaction bottle, add minocycline hydrochloride (100g) at temperature control -20°C, and stir until it dissolves. Potassium nitrate (24.3 g) was added slowly and uniformly at a temperature of -20°C for 2.5 hours. After adding and keeping warm for 1 h, HPLC detection showed that the purity was 89.8%, and the C4-epimer was 0.2%. Control the temperature at -5°C and slowly add 10% ammonia methanol solution dropwise until no precipitation occurs, filter, and the filtrate is 9-nitrominocycline methanol solution. HPLC detection shows that the purity is 93.2%. 9-Nitrocycline C4-epimer of Kiminocycline 0.2%. The filtrate was concentrated to dryness under reduced pressure to obtain a solid, which was detected by HPLC, showing that the purity was 93.2%, and the C4-epimer of 9-nitrominocycline was 0.2%, which had no significant difference from that in the solution. The resulting solid residue on ignition was 0.05%.
[0036] It can b...
Embodiment 2
[0038] Add concentrated sulfuric acid (180ml) into the reaction bottle, add minocycline hydrochloride (90g) at temperature control -5°C, and stir until it dissolves. Potassium nitrate (25 g) was added slowly and uniformly at a temperature of -5°C for 2.5 hours. After adding and keeping warm for 1 hour, HPLC detection showed that the purity was 88.5%, and the C4-epimer of 9-nitrominocycline was 0.2%. Control the temperature at 0°C and slowly add 10% methanol solution of ammonia gas dropwise until no more precipitation occurs, filter, and the filtrate is 9-nitrominocycline methanol solution. HPLC detection shows that the purity is 92.1%, 9-nitrominocycline C4-epimer of minocycline 0.2%. The filtrate was concentrated to dryness under reduced pressure to obtain a solid, which was detected by HPLC, showing a purity of 92.0%, and a C4-epimer of 9-nitrominocycline of 0.2%. The resulting solid residue on ignition was 0.06%.
Embodiment 3
[0040] Add concentrated sulfuric acid (180ml) into the reaction bottle, add minocycline hydrochloride (60g) at -20°C under temperature control, and stir until it dissolves. Potassium nitrate (17 g) was added slowly and uniformly at a temperature of -15°C for 2.2 hours. After adding and keeping warm for 1 hour, HPLC detection showed that the purity was 89.1%, and the C4-epimer of 9-nitrominocycline was 0.2%. Control the temperature at -5°C and slowly add 10% ammonia methanol solution dropwise until no precipitation occurs, filter, and the filtrate is 9-nitrominocycline methanol solution. HPLC detection shows that the purity is 92.3%. 9-Nitrocycline C4-epimer of Kiminocycline 0.2%. The filtrate was concentrated to dryness under reduced pressure to obtain a solid, which was detected by HPLC, showing a purity of 92.4%, and a C4-epimer of 9-nitrominocycline of 0.2%. The resulting solid residue on ignition was 0.04%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com