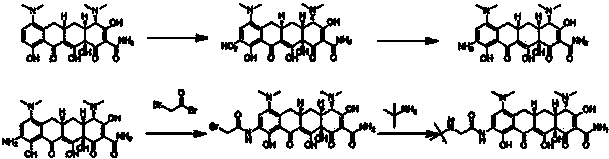
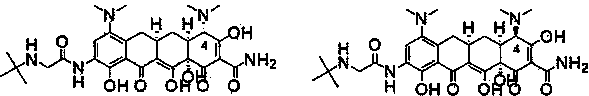Production method of tigecycline raw medicinal material
A technology for tigecycline and a production method, which is applied in the directions of chemical instruments and methods, anti-infective drugs, drug combinations, etc., can solve the problems of increasing the impurity content of isomers, affecting product quality, and difficult to separate by-products, and achieves overcoming The effect of high difficulty, improved quality, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
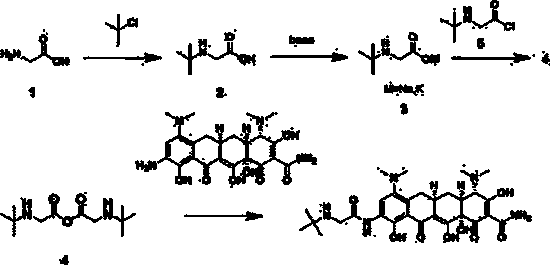
[0033] Add glycine (10.00g, 0.13mol) and triethylamine (15.00g, 0.15mol) into dichloromethane (50ml), stir under ice bath until the temperature of the system is below 5°C, at this temperature the above system drops 2-Chloro-2-methylpropane (14.00 g, 0.15 mol) diluted with dichloromethane (20 mL) was added. After the dropwise addition, react for 1 h. The pH was adjusted to 10 with sodium hydroxide solution, and the reaction was stirred at room temperature for 30 min. Add hydrochloric acid to the reaction system, adjust the pH to 5 and stir at room temperature for 1 h. Extract with ethyl acetate. The organic phase was dried over anhydrous sodium sulfate, distilled under reduced pressure and dried to obtain compound 2 (16.59 g), with a yield of 95%.
Embodiment 2
[0035] Add glycine (10.00g, 0.13mol) and triethylamine (20.21g, 0.20mol) into dichloromethane (50ml), stir under ice bath until the temperature of the system is below 5°C, at this temperature the above system drops Add 2-chloro-2-methylpropane (18.49g, 0.20mol) diluted with dichloromethane (20ml). After the dropwise addition, react for 1 h. The pH was adjusted to 10 with sodium hydroxide solution, and the reaction was stirred at room temperature for 30 min. Add hydrochloric acid to the reaction system, adjust the pH to 5 and stir at room temperature for 1 h. Extract with ethyl acetate. The organic phase was dried with anhydrous sodium sulfate, distilled under reduced pressure and dried to obtain compound 2 (16.86 g), with a yield of 96.5%.
Embodiment 3
[0037] Compound 2 (16.59 g, 0.13 mol) and potassium hydroxide (7.10 g, 0.13 mol) were added to isopropanol. Heat to reflux for 3h, cool and filter, and wash the filter cake with an appropriate amount of dichloromethane. After drying, the crude product (21.32 g) was obtained and used directly in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com