Preparation method of tigecycline composition
A technology of tigecycline and its composition, which is applied in the field of pharmaceutical preparations, can solve the problems of easily causing allergic reactions, etc., and achieve the effects of easy industrial operation, stable sample quality, low protein content and bacterial endotoxin content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Tigecycline 50g
[0022] Lactose 100g
[0023] Add water for injection to 2000ml
[0024] Freeze-dried to make 1000 vials
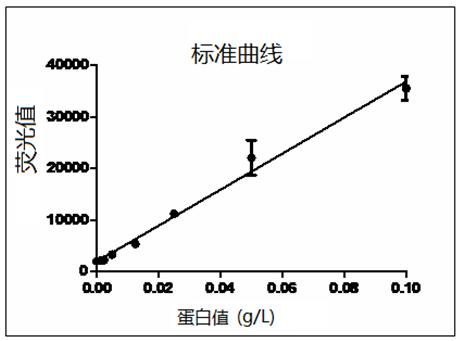
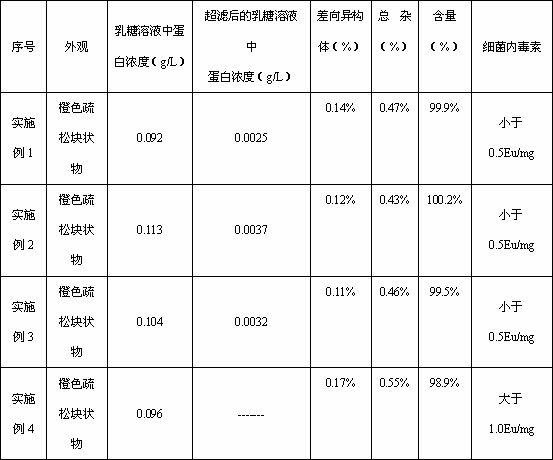
[0025] Weigh lactose and put it in a sterile container, add 80% water for injection to dissolve it, and carry out ultrafiltration treatment on the lactose solution, collect the ultrafiltrate, after the solution drops to 4°C, add tigecycline, dissolve and stir Evenly, adjust the pH to 5.0 with 1mol / L hydrochloric acid or 1mol / L sodium hydroxide solution, add water for injection to the full amount, then add 0.1% activated carbon for needles, stir and adsorb for 20 minutes, and remove carbon by suction filtration; after the intermediate inspection is qualified, The solution is filtered through two 0.22m microporous membranes and then sent to the sterile room, filled in vials, half-plugged with butyl rubber stoppers, placed in a plate, placed in a freeze-drying box, closed the box door, and turned on for refrigeration , using heat...
Embodiment 2
[0027] Tigecycline 50g
[0028] Lactose 200g
[0029] Add water for injection to 2000ml
[0030] Freeze-dried to make 1000 vials
[0031] Weigh lactose and put it in a sterile container, add 80% water for injection to dissolve it, and carry out ultrafiltration treatment on the lactose solution, collect the ultrafiltrate, after the solution drops to 6°C, add tigecycline, dissolve and stir Evenly, adjust the pH to 5.5 with 1mol / L hydrochloric acid or 1mol / L sodium hydroxide solution, add water for injection to the full amount, then add 0.1% activated carbon for needles, stir and adsorb for 20 minutes, and remove carbon by suction filtration; after the intermediate inspection is qualified, The solution is filtered through two 0.22m microporous membranes and then sent to the sterile room, filled in vials, half-plugged with butyl rubber stoppers, placed in a plate, placed in a freeze-drying box, closed the box door, and turned on for refrigeration , using heat...
Embodiment 3
[0033] Tigecycline 50g
[0034] Lactose 150g
[0035] Add water for injection to 2000ml
[0036] Freeze-dried to make 1000 vials
[0037] Weigh lactose and put it in a sterile container, add 80% water for injection to dissolve it, and carry out ultrafiltration treatment on the lactose solution, collect the ultrafiltrate, after the solution drops to 4°C, add tigecycline, dissolve and stir Evenly, adjust the pH to 4.5 with 1mol / L hydrochloric acid or 1mol / L sodium hydroxide solution, add water for injection to the full amount, then add 0.1% activated carbon for needles, stir and absorb for 20 minutes, and remove carbon by suction filtration; after the intermediate inspection is qualified, The solution is filtered through two 0.22m microporous membranes and then sent to the sterile room, filled in vials, half-plugged with butyl rubber stoppers, placed in a plate, placed in a freeze-drying box, closed the box door, and turned on for refrigeration , using heat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com