Application of 2,6-bis(2-benzimidazolyl) pyridine in preparation of carbapenem-resistant pseudomonas aeruginosa infection drug
A Pseudomonas aeruginosa, benzimidazole-based technology, applied in the field of medicine, can solve the problems of decreased drug concentration, bacteria cannot be effectively killed, etc., and achieves the effect of reducing bacteriostatic concentration, high purity of synthetic products and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
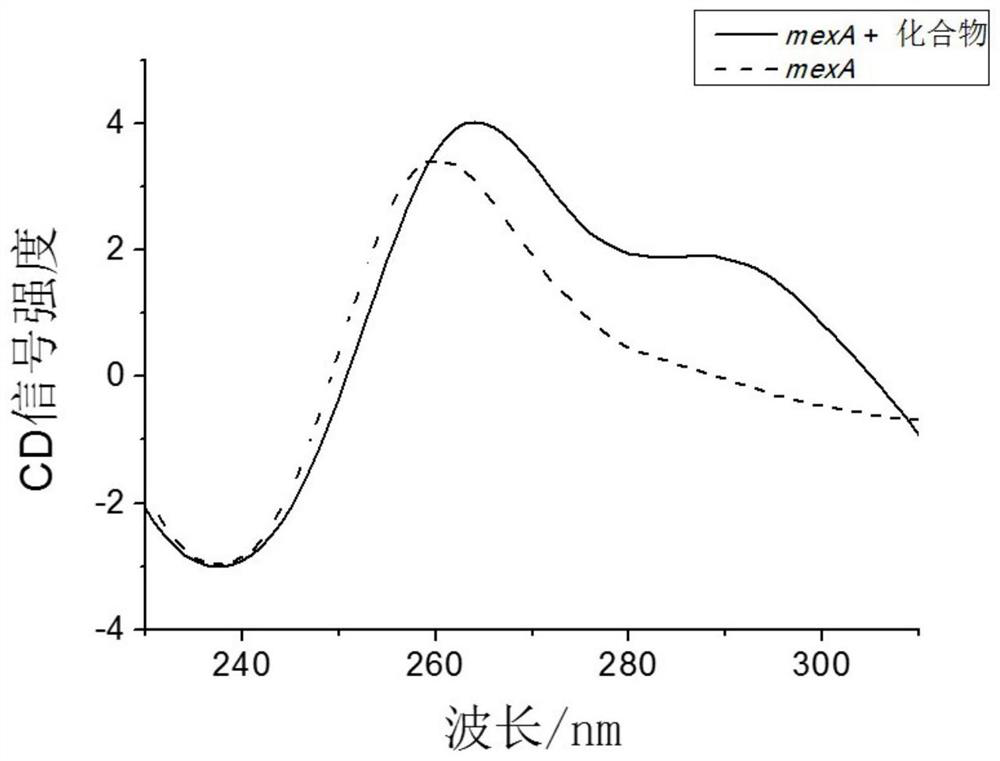
[0020] Example 1: Verification that 2,6-bis(2-benzimidazolyl)pyridine induces the core sequence of MexA gene to form a G-quadruplex structure.
[0021] 1. Experimental equipment
[0022] The circular dichroism spectrometer was purchased from Applied Optical Physics, UK
[0023] 2. Experimental drugs and reagents
[0024] 2,6-bis(2-benzimidazolyl)pyridine was purchased from Alan (Shanghai) Huagong Technology Co., Ltd., the short-chain MexA core sequence was obtained from GeneBank (ID: 877855), and the DNA was purchased from Sangon Bioengineering (Shanghai) ) Co., Ltd., the sequence is 5'-GGCGGCGGTGGAGCAG-3', trimethylaminomethane (Tris) was purchased from Huamei Bioengineering Company, and the high-concentration stock solution of 2,6-bis(2-benzimidazolyl)pyridine was 20mM, dissolved in DMSO.
[0025] 3. Experimental method
[0026] System 1: Contains 10 μM MexA DNA and 10 mM Tris-HCl at a final concentration of 200 μL in total, and fill up the deficiency with distilled wate...
Embodiment 2
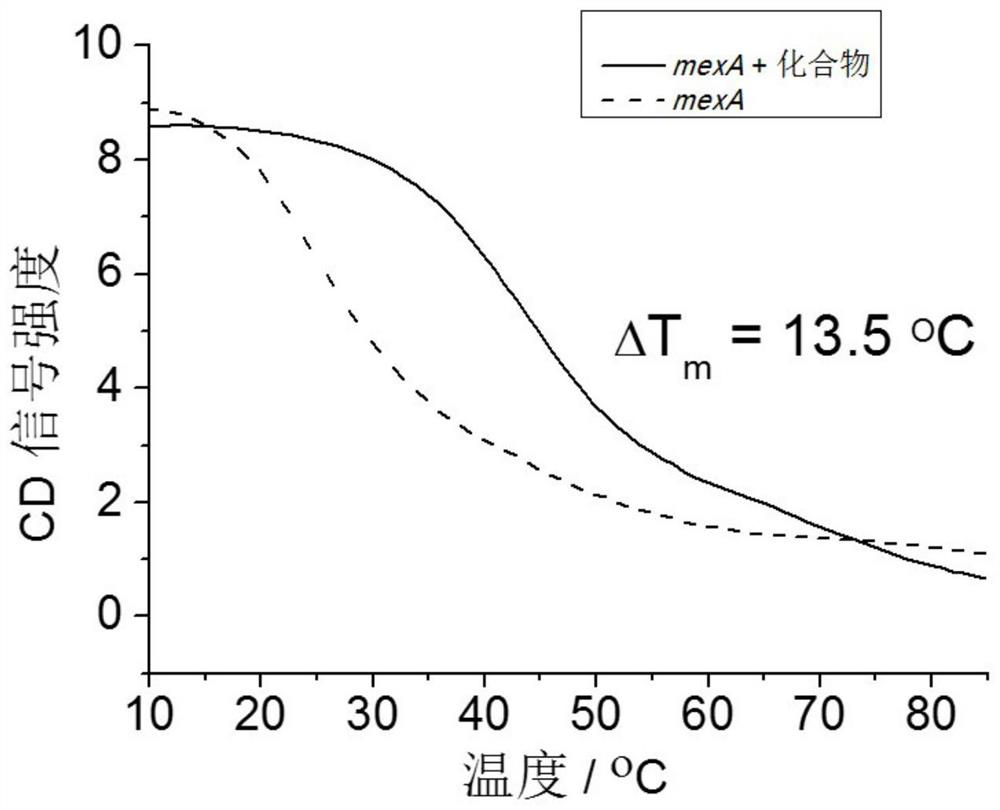
[0031] Example 2: Stability verification of the G-quadruplex structure formed by the core sequence of the MexA gene induced by 2,6-bis(2-benzimidazolyl)pyridine.
[0032] 1. Experimental equipment
[0033] The circular dichroism spectrometer was purchased from Applied Optical Physics, UK
[0034] 2. Experimental drugs and reagents
[0035] 2,6-bis(2-benzimidazolyl)pyridine was purchased from Alan (Shanghai) Huagong Technology Co., Ltd., the short-chain MexA core sequence was obtained from GeneBank (ID: 877855), and the DNA was purchased from Sangon Bioengineering (Shanghai) ) Co., Ltd., the sequence is 5'-GGCGGCGGTGGAGCAG-3', trimethylaminomethane (Tris) was purchased from Huamei Bioengineering Company, and the high-concentration stock solution of 2,6-bis(2-benzimidazolyl)pyridine was 20mM, dissolved in DMSO.
[0036] 3. Experimental method
[0037] System 1: Containing a final concentration of 20 μM MexA DNA and 10 mM Tris-HCl, the total volume is 200 μL, and the insuffic...
Embodiment 3
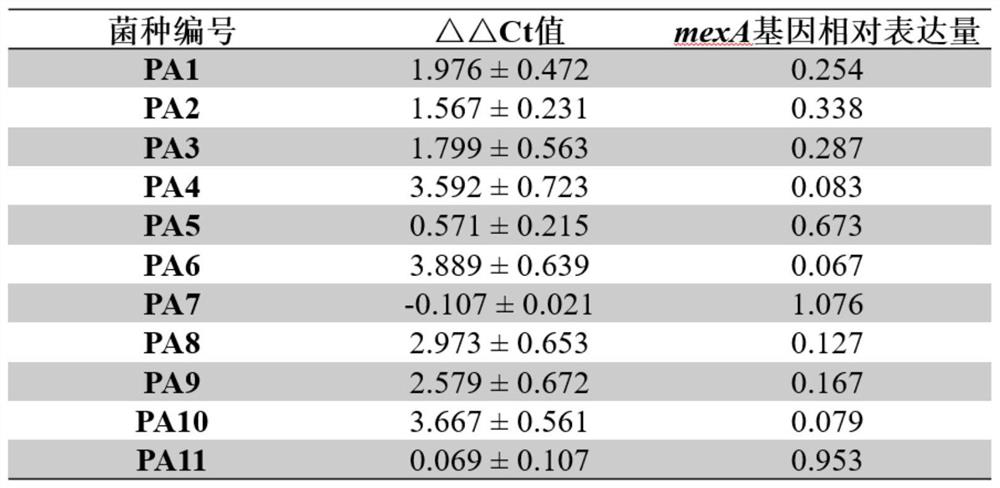
[0042] Example 3: Validation of 2,6-bis(2-benzimidazolyl)pyridine inhibiting MexA gene expression in carbapenem-resistant Pseudomonas aeruginosa.
[0043] 1. Experimental equipment
[0044] Fluorescent quantitative PCR instrument was purchased from Bio-Rad Company in the United States, Nanodrop2000 ultra-micro tube luminosity reagent was purchased from Thermo Fisher Corporation in the United States, and the water-proof constant temperature incubator was purchased from Shanghai Shuli Instrument Co., Ltd.
[0045] 2. Strains
[0046] Collect Pseudomonas aeruginosa strains isolated from a hospital microbiology laboratory from July to December 2018, and exclude repeated strains from the same patient. version) identified carbapenem-resistant Pseudomonas aeruginosa strains, and collected 11 carbapenem-resistant Pseudomonas aeruginosa strains. The quality control strain was Pseudomonas aeruginosa ATCC27853, which was purchased from Guangdong Huankai Microbial Technology Co., Ltd. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com