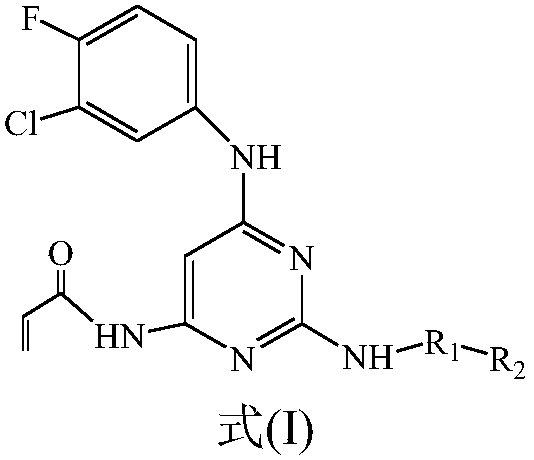
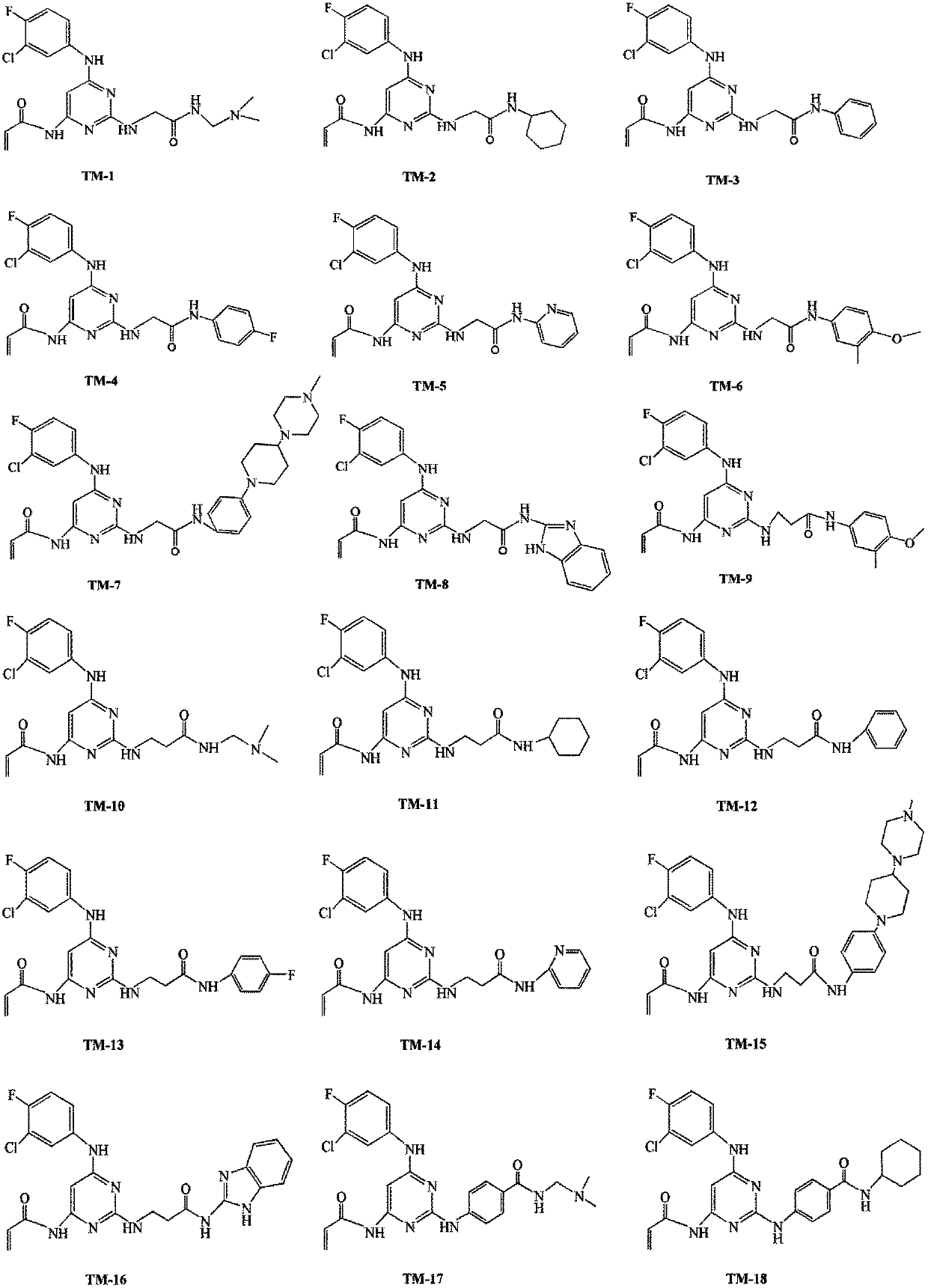
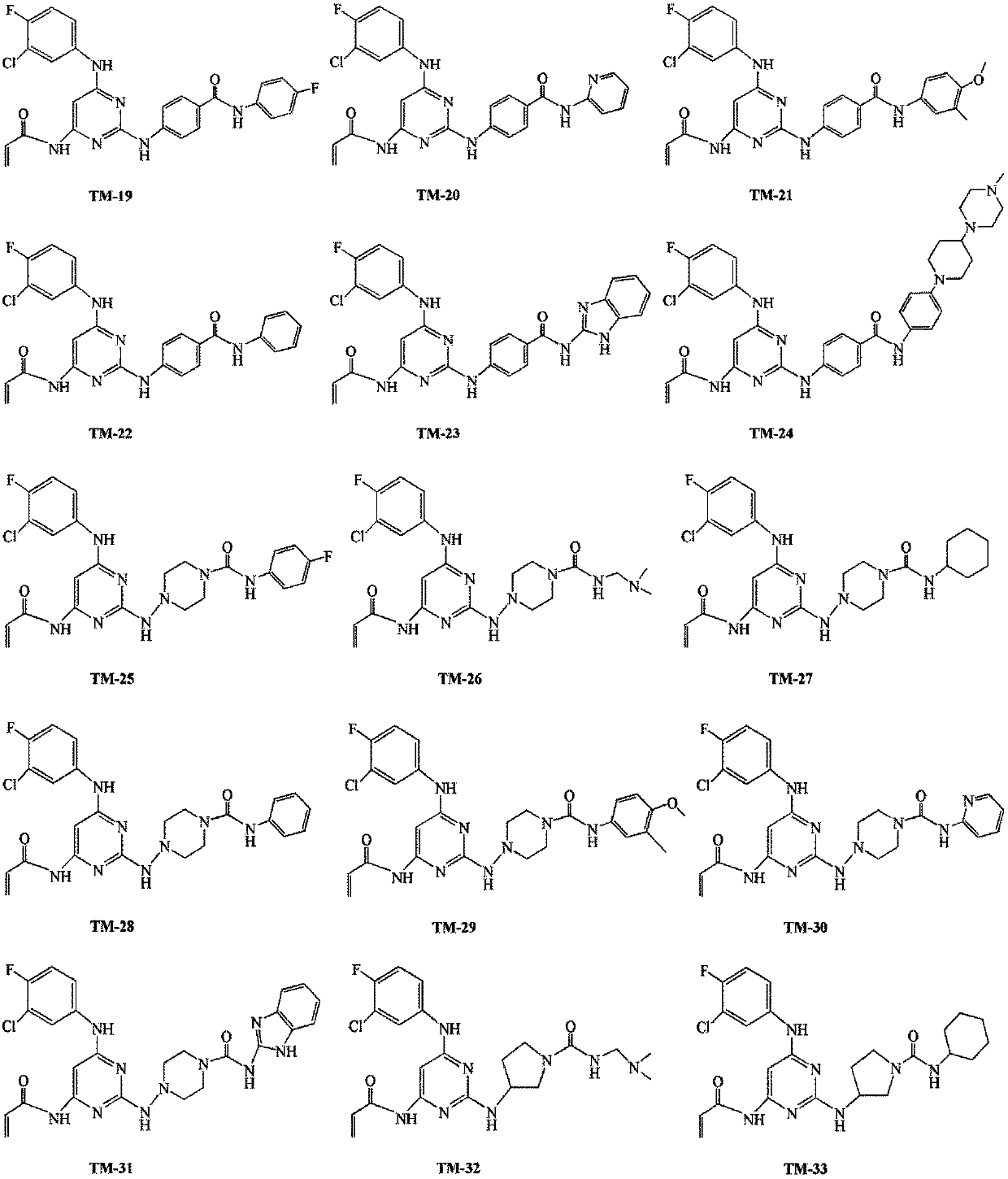
Amido pyrimidine compound
A technology of aminopyrimidines and compounds, applied in the field of preparation of 2, inhibitors, can solve the problem of incomplete solution of toxic and side effects, achieve good cytotoxicity, improve solubility, and increase affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] The preparation of embodiment 1 TM-1
[0140]
[0141] Compound 3: Glycine (21.03g, 120mmol) protected by tert-butoxycarbonyl was added to dichloromethane (30ml), and EDCI (25.31g, 132mmol), HOBt (17.82g, 132mmol) and N, N- Dimethylethylenediamine (11.63g, 132mmol), stirred at room temperature for 36h, TLC detected the end of the reaction, removed the solvent under reduced pressure, and redissolved the obtained solid in 120mL mixed solution (DCM:TFA=7:3), stirred at room temperature for reaction After 0.5 h, TLC detected that the reaction was complete, and the solvent was removed by rotary evaporation under reduced pressure, and the product compound 3 was separated by column chromatography with a yield of 76.42%, and HPLC: 99.56%.
[0142]Intermediate III: Add 2,4,6-trichloropyrimidine (Compound 1) (55.03g, 300mmol) into dichloromethane (300ml) and stir until dissolved, cool down to 0-5°C in an ice bath, slowly add 3 -Chloro-4-fluoroaniline (45.71g, 315mmol, compoun...
Embodiment 2
[0146] The preparation of embodiment 2 TM-2
[0147]
[0148] Compound 3: Glycine (21.03g, 120mmol) protected by tert-butoxycarbonyl group was added in dichloromethane (40ml), and EDCI (25.31g, 132mmol), HOBt (17.82g, 132mmol) and cyclohexylamine ( 13.09g, 132mmol), stirred at room temperature for 24h, TLC detected the end of the reaction, removed the solvent under reduced pressure, and redissolved the obtained solid in 150mL mixed solution (DCM:TFA=7:3), stirred at room temperature for 0.5h, and detected the reaction by TLC At the end, the solvent was removed by rotary evaporation under reduced pressure, and the product compound 3 was separated by column chromatography with a yield of 73.62%, HPLC: 99.62%.
[0149] Intermediate III: Add 2,4,6-trichloropyrimidine (compound 1) (55.03g, 300mmol) into ethyl acetate (300ml) and stir until dissolved, cool down to 0-5°C in an ice bath, slowly add 3 -Chloro-4-fluoroaniline (45.72g, 315mmol, compound 2), the ice bath was removed a...
Embodiment 3
[0153] The preparation of embodiment 3 TM-3
[0154]
[0155] Compound 3: Glycine (21.03g, 120mmol) protected by tert-butoxycarbonyl was added to dichloromethane (30ml), and EDCI (25.31g, 132mmol), HOBt (17.82g, 132mmol) and aniline (12.29g) were added successively , 132mmol), stirred at room temperature for 24h, TLC detected the end of the reaction, removed the solvent under reduced pressure, and the resulting solid was redissolved in 120mL mixed solution (DCM:TFA=7:3), stirred at room temperature for 0.5h, and TLC detected the end of the reaction. The solvent was removed by rotary evaporation under reduced pressure, and the product compound 3 was separated by column chromatography with a yield of 75.61%, HPLC: 99.71%.
[0156] Intermediate III: Add 2,4,6-trichloropyrimidine (compound 1) (55.03g, 300mmol) into acetone (300ml) and stir until dissolved, cool down to 0-5°C in an ice bath, and slowly add 3-chloropyrimidine dropwise -4-Fluoroaniline (45.72g, 315mmol, compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com