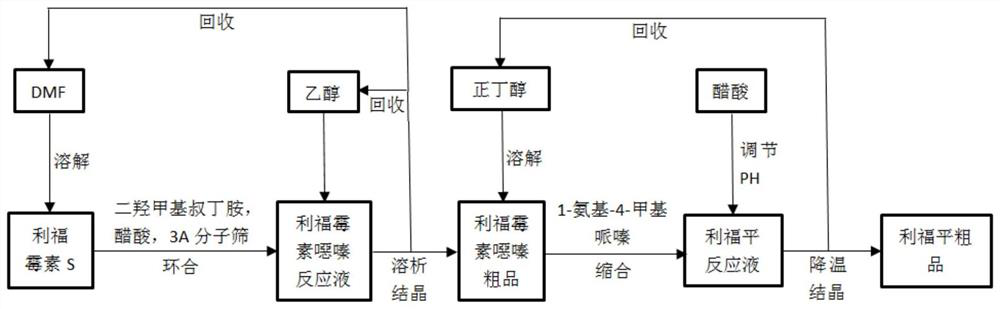
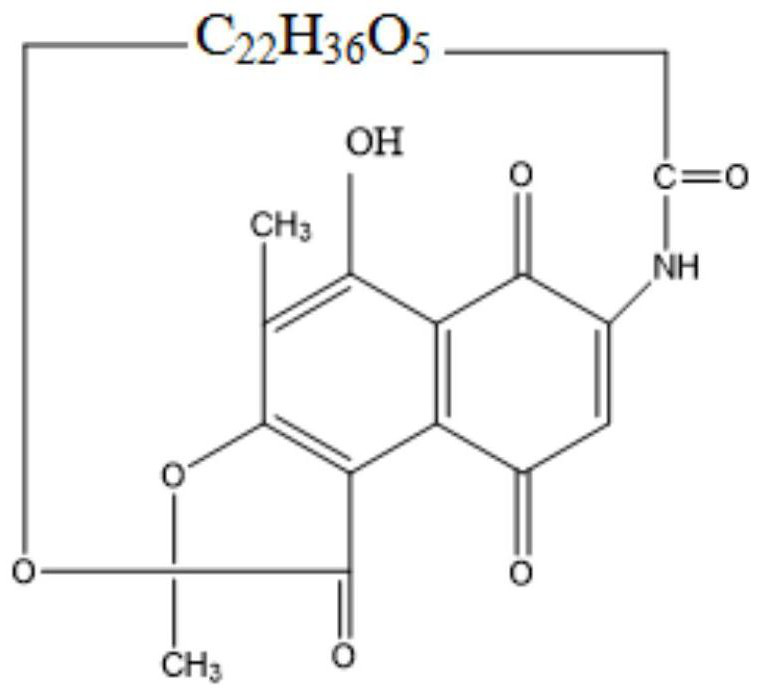
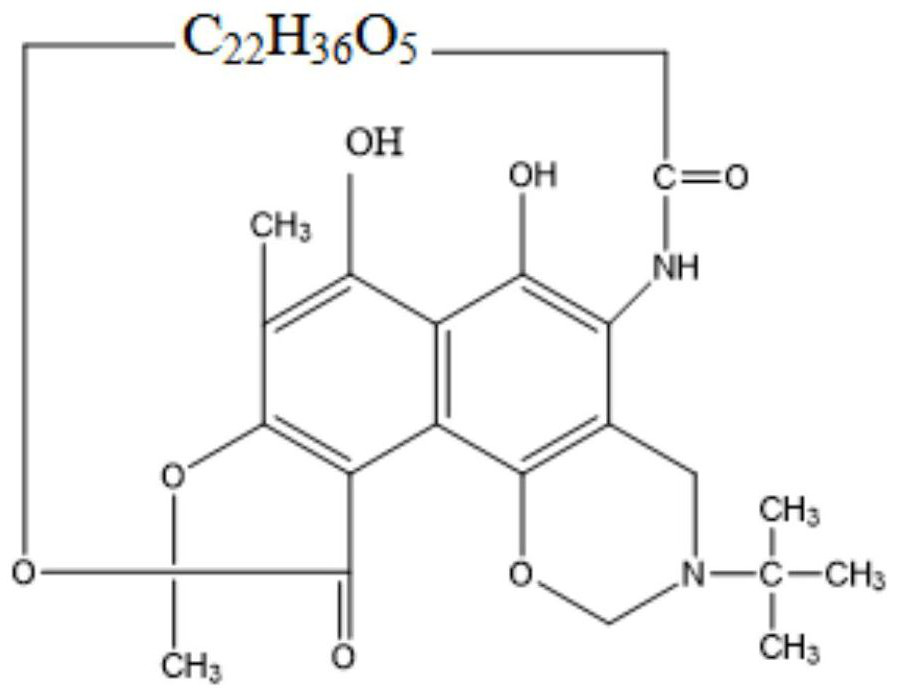
Process method for synthesizing rifampicin
A process method, rifampicin technology, applied in the field of compound preparation, can solve the problems of difficult source of raw materials, high toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] A process for synthesizing rifampicin, comprising the following steps:
[0061] (1) Cyclization reaction: Take 1.0kg of rifamycin S in a three-necked jacketed bottle, add DMF to dissolve under stirring, heat in a constant temperature water bath and maintain at 55°C, add acetic acid and 3A molecular sieve, add dimethylol tert-butylamine, the reaction time is 90min, and the ratio of each material is V (DMF): V (dimethylol tert-butylamine): V (acetic acid): M (3A molecular sieve): M (rifamycin S) = 1.0L: 0.2 L: 0.4L: 0.06kg: 1.0kg.
[0062] (2) Dissolution and crystallization conditions: after the cyclization reaction, ethanol preheated to 40° C. was slowly added to the reaction solution. The amount of ethanol added was 9.0 L / kg rifamycin S, and the volume fraction of DMF was 10%. Cool down and crystallize under the stirring state, turn off the stirring when the temperature drops to 5°C, let it stand for 2 hours, and then filter with suction to obtain the crude product of...
Embodiment 2
[0066] A kind of processing method of synthesizing rifampicin, reference figure 1 , including the following steps:
[0067] (1) Cyclization reaction: Take 5g of rifamycin S in a 150ml three-necked jacketed bottle, add a certain volume of DMF to dissolve it under stirring, heat it in a constant temperature water bath and maintain it at 60°C, add a certain amount of acetic acid and 3A Molecular sieve, add dimethylol tert-butylamine, the reaction time is 80min, in the cyclization reaction, each material ratio is V (DMF): V (dimethylol tert-butylamine): V (acetic acid): M (3A molecular sieve): M ( Rifamycin S)=(1L):(0.2L):(0.4L):(0.06kg):(1.0kg).
[0068] (2) Dissolution and crystallization conditions: after the cyclization reaction, ethanol preheated to 20° C. was slowly added to the reaction solution, the amount of ethanol added was 19 L / kg rifamycin S, and the volume fraction of DMF was 5%. Cool down and crystallize under the stirring state, turn off the stirring when the te...
Embodiment 3
[0072] A kind of processing method of synthesizing rifampicin, reference figure 1 , including the following steps:
[0073] (1) Cyclization reaction: Take rifamycin S 5g in a 150ml three-necked jacketed bottle, add a certain volume of DMF to dissolve it under stirring, heat it in a constant temperature water bath and maintain it at 40°C, add a certain amount of acetic acid and 3A Molecular sieve, add dimethylol tert-butylamine, the reaction time is 70min, in the cyclization reaction, each material ratio is V (DMF): V (dimethylol tert-butylamine): V (acetic acid): M (3A molecular sieve): M ( Rifamycin S)=(1L):(0.2L):(1L):(0.06kg):(1.0kg).
[0074] (2) Dissolution and crystallization conditions: after the cyclization reaction, slowly add ethanol preheated to 30°C to the reaction solution, the amount of ethanol added is 9L / kg rifamycin S, and the volume fraction of DMF is 10%. Cool down and crystallize under agitation, turn off the agitation when the temperature drops to 5°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com