Method for continuously preparing benzoxazine rifamycin
A technology for rifamycin and benzoxazine, which is applied in the field of continuous preparation of benzoxazine-rifamycin, can solve the problems of low reaction yield, poor operation stability, large amount of solvent and the like, achieves high yield, Improved process efficiency and excellent mixing results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
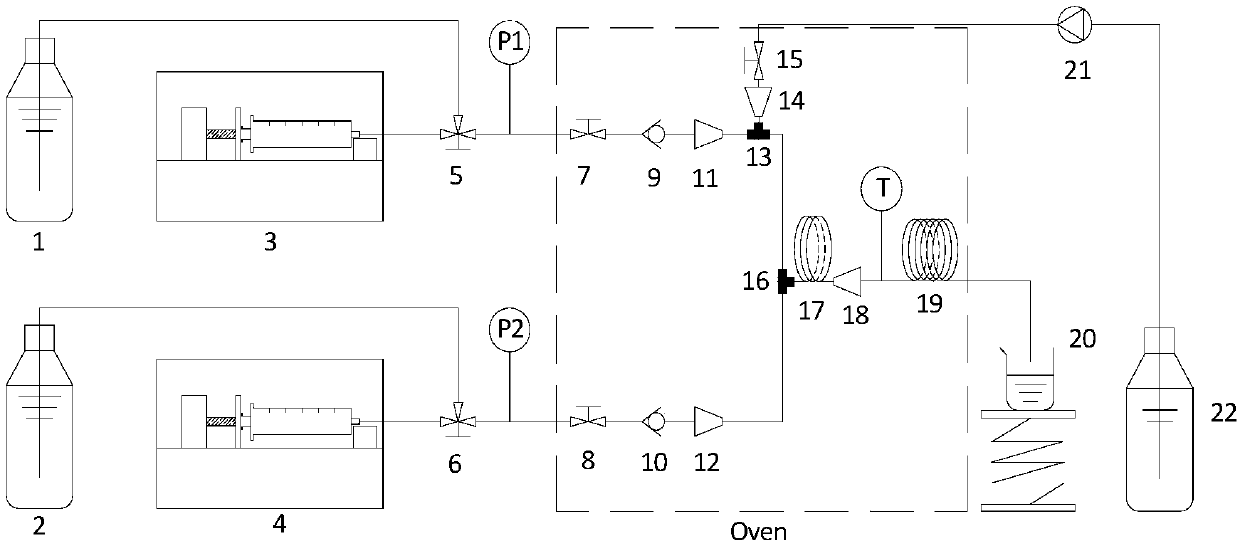
[0025] Use N,N-dimethylformamide as solvent to prepare 0.394M rifamycin S solution 1 and 1.362M dimethylol-tert-butylamine solution 2, and both materials pass through the metering pump 3 and metered at a flow rate of 0.3mL / min. The pump 4 is transported to the micro-mixer 16 through two-way ball valves 7, 8, one-way valves 9, 10 and variable diameter joints 11, 12, and then enters the micro-reactor system to start the reaction. The reaction temperature is 80°C, and the reaction residence time is After 32.3 minutes, the crude product was collected at the outlet, and after rotary steaming, the sample was analyzed by HPLC. The conversion rate of rifamycin S was 98.2%, and the yield of benzoxazine rifamycin was 78.1%.
Embodiment 2
[0027] Use N,N-dimethylformamide as solvent to prepare 0.386M rifamycin S solution 1 and 1.362M dimethylol-tert-butylamine solution 2, and both materials pass through the metering pump 3 and metered at a flow rate of 0.3mL / min. Pump 4, through two-way ball valves 7,8, one-way valves 9,10 and reducing joints 11,12, is transported to the micro-mixer 16 and then enters the micro-reactor system to start the reaction. The reaction temperature is 70°C, and the reaction residence time is After 32.3 minutes, the crude product was collected at the outlet, and after rotary steaming, the sample was analyzed by HPLC. The conversion rate of rifamycin S was 98.9%, and the yield of benzoxazine rifamycin was 95.9%.
Embodiment 3
[0029] Use N,N-dimethylformamide as solvent to prepare 0.392M rifamycin S solution 1 and 1.362M dimethylol-tert-butylamine solution 2, and both materials pass through metering pump 3 and metered at a flow rate of 0.242mL / min. Pump 4, through two-way ball valves 7,8, one-way valves 9,10 and reducing joints 11,12, is transported to the micro-mixer 16 and then enters the micro-reactor system to start the reaction. The reaction temperature is 70°C, and the reaction residence time is After 40 minutes, the crude product was collected at the outlet, and after rotary steaming, the sample was analyzed by HPLC. The conversion rate of rifamycin S was 98.3%, and the yield of benzoxazine rifamycin was 93.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com