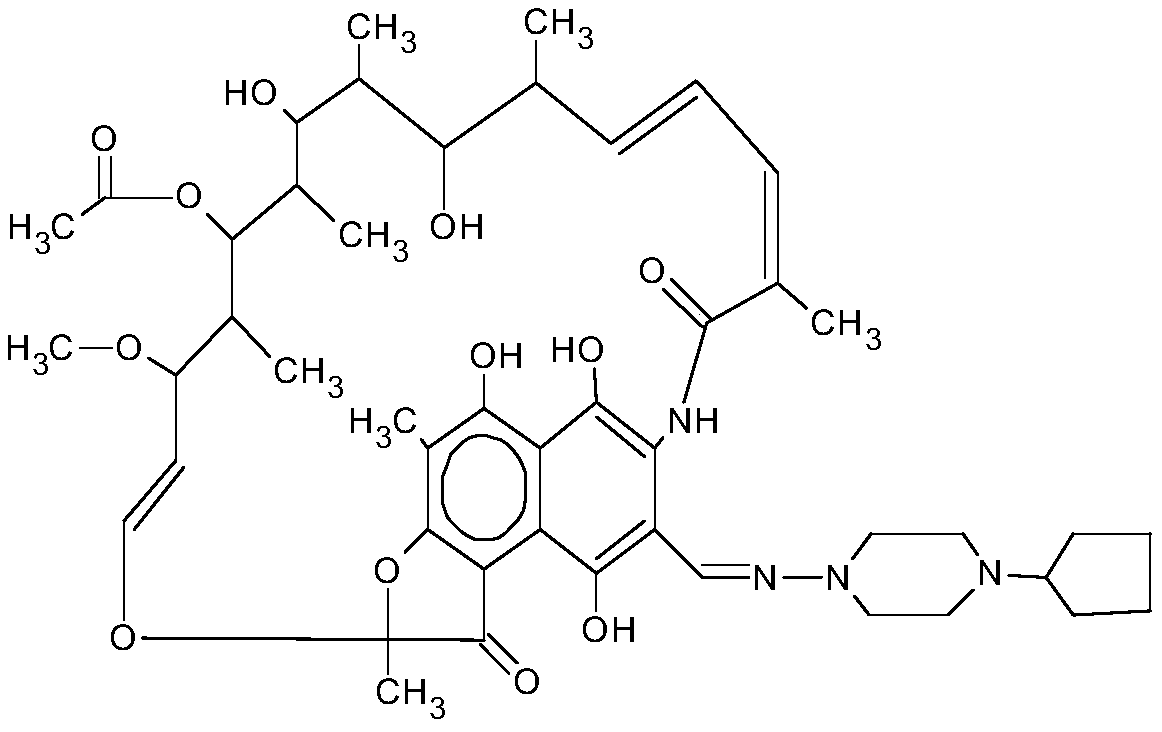
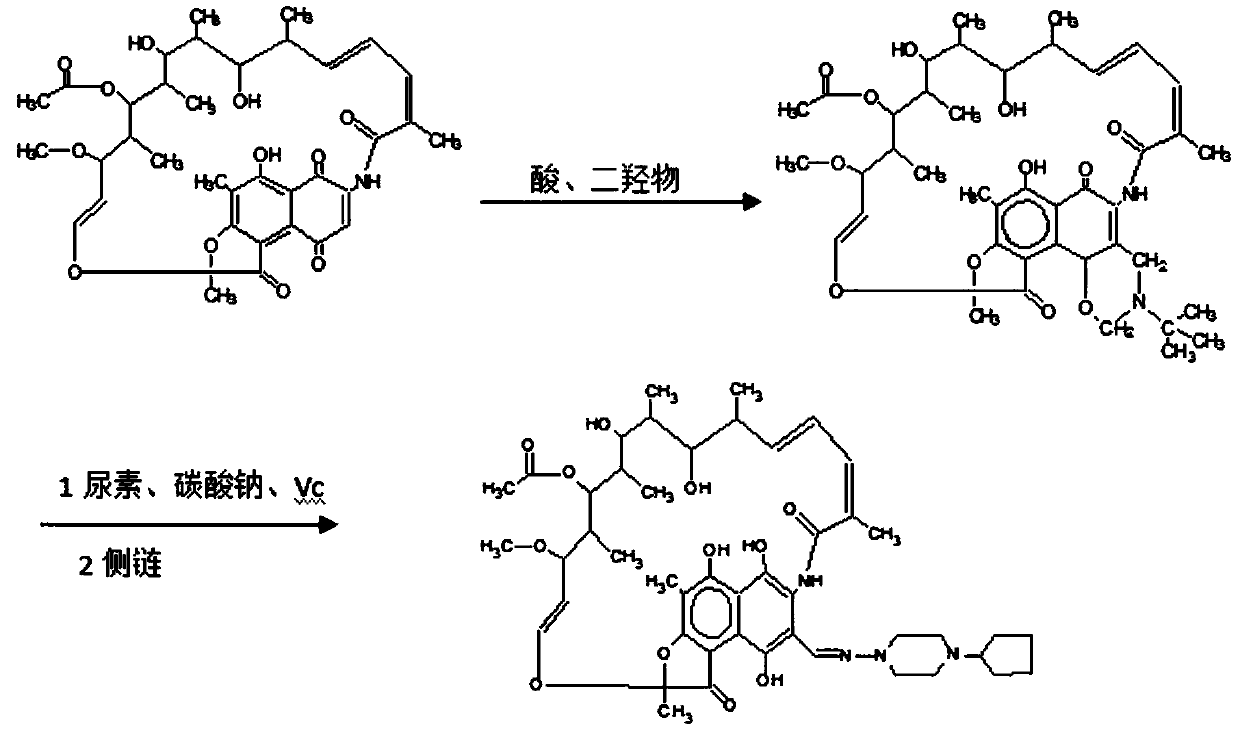
Preparation method of high-purity rifapentine
A rifapentine, high-purity technology, applied in the field of medicine, can solve the problems of insufficient rifapentine purity and unsatisfactory use effect, and achieve the effects of improving stability, reducing production, and increasing speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The present invention will be described in detail below in conjunction with various embodiments, but it should be noted that these embodiments are not limitations of the present invention, and those of ordinary skill in the art can make functional, method, or structural equivalent transformations or replacements based on these embodiments. , all fall within the protection scope of the present invention.
[0025] This embodiment discloses a preparation method of high-purity rifapentine, comprising the following steps:
[0026] Step 1: cyclization reaction of rifamycin S acid, acid, and dimethylol terbumine in N,N-dimethylformamide solvent; the amount of acid in the cyclization reaction is the amount of rifamycin S acid 0.3 to 0.4 times (v / w), the pH value is under acidic conditions, and the ring-opening reaction is a two-way reaction, which is beneficial to the forward reaction in an acidic synthesis environment and is helpful to the improvement of purity.
[0027] Step...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com