Method for preparing rifampicin by utilizing cascade reaction of kettle type reaction device and microchannel reaction device
A technology of microchannel reaction and reaction device, which is applied in chemical instruments and methods, chemical/physical/physical chemical reactors, chemical/physical/physical chemical processes, etc. Achieve the effect of saving raw material cost, short reaction time and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
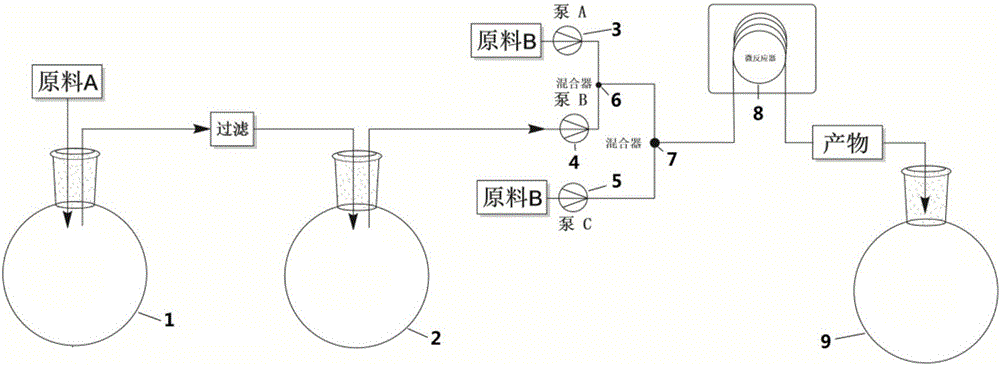
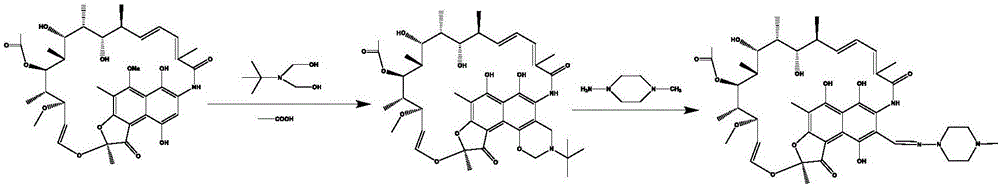
[0032] Take rifamycin S sodium salt (42g, 0.0584mol) with a purity of 95% and place it in a three-necked flask A, add 70mL N,N-dimethylformamide and sulfuric acid (2.84mL, 0.0533mol), and stir at room temperature for 45min , the reaction solution was filtered; after the filtration was completed, the filtrate was placed in a three-necked beaker bottle B, dimethylol terbutylamine (9.86mL, 0.0759mol) was added, and stirred at 45°C for 2h; 1-methyl-4- Amino-piperazine (10mL, 0.086mol) was placed in a three-necked flask C, and 193mL of N,N-dimethylformamide was added, and stirred to form a homogeneous solution; pump A was used to extract acetic acid and N,N with a volume ratio of 1:100 -The mixture of dimethylformamide, the flow rate is 0.0436mL / min, the reaction solution in the three-necked flask B is directly extracted by the pump B, the flow rate is 0.623mL / min, and the two are mixed with a T-type mixing valve; Extract the liquid in the three-necked flask C at a flow rate of 1.3...
Embodiment 2
[0034] Take rifamycin S sodium salt (42 g, 0.0584 mol) with a purity of 95% and place it in a three-necked flask A, add 70 mL N, N-dimethylformamide and sulfuric acid (2.84 mL 0.0533 mol), and stir at room temperature for 45 min. The reaction solution was filtered; after the filtration was completed, the filtrate was placed in a three-necked beaker bottle B, dimethylol terbutylamine (9.86mL 0.0759mol) was added, and stirred at 45°C for 2h; 10mL 0.086mol1-methyl-4- Amino-piperazine was placed in a three-necked flask C, and 193mL N,N-dimethylformamide was added, and stirred to form a homogeneous solution; pump A pumped acetic acid and N,N-dimethylformamide with a volume ratio of 1:100 The mixture, the flow rate is 0.117mL / min, the reaction solution in the three-necked flask B is directly extracted by the pump B, the flow rate is 1.68mL / min, and the two are mixed with a T-shaped mixing valve; the reaction solution in the three-necked flask C is directly extracted by the pump C Th...
Embodiment 3
[0036] Take rifamycin S sodium salt (42g, 0.0584mol) with a purity of 95% and place it in a three-necked flask A, add 70mL N,N-dimethylformamide and (2.84mL 0.0533mol) sulfuric acid, and stir at 40°C for 1h , filter the reaction solution; after the filtration is completed, put the filtrate into a three-necked beaker bottle B, add (9.86mL 0.0759mol) dimethylol terbutylamine, and stir at 45°C for 2h; add 10mL0.086mol 1-methyl- 4-Amino-piperazine was placed in a three-necked flask C, and 193mL N,N-dimethylformamide was added, and stirred to form a homogeneous solution; pump A was used to extract acetic acid and N,N-dimethylformamide with a volume ratio of 1:100 A mixture of formamide, the flow rate is 0.0436mL / min, the reaction solution in the three-necked flask B is directly extracted by the pump B, the flow rate is 0.623mL / min, and the two are mixed with a T-shaped mixing valve; the three-necked flask C is directly extracted by the pump C The liquid in the medium, with a flow r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com